当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling P–C/C–H Bond Cleavage in Nickel Bis(diphosphine) Complexes: Reactivity Scope, Mechanism, and Computations
Organometallics ( IF 2.5 ) Pub Date : 2020-09-03 , DOI: 10.1021/acs.organomet.0c00388 Shaoguang Zhang 1 , Haixia Li 2 , Aaron M. Appel 1 , Michael B. Hall 2 , R. Morris Bullock 1
Organometallics ( IF 2.5 ) Pub Date : 2020-09-03 , DOI: 10.1021/acs.organomet.0c00388 Shaoguang Zhang 1 , Haixia Li 2 , Aaron M. Appel 1 , Michael B. Hall 2 , R. Morris Bullock 1
Affiliation
|
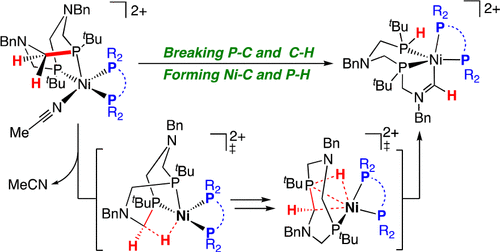
The synthesis of heteroleptic [Ni(P2N2)(diphosphine)][BF4]2 complexes and the cleavage of P–C and C–H bonds of the P2N2 ligand in those complexes are reported. The products are five-coordinate complexes in which Ni–C and P–H bonds have formed to give a cyclic moiety containing Ni–CH═NR2. The reactivity of [Ni(P2N2)(diphosphine)][BF4]2 complexes is influenced by the rigidity of the diphosphine, the steric effect of the substituents, and length of the carbon linker of the diphosphine ligands. Diphosphine ligands bearing a rigid backbone (e.g., dmpbz, 1,2-bis(dimethylphosphino)benzene) or aromatic substituents (e.g., dppe, 1,2-bis(diphenylphosphino)ethane) react with [Ni(PtBu2NBn2)(CH3CN)2][BF4]2 to give P–C/C–H bond cleavage products. Both [Ni(PtBu2NBn2)(dmpe)(MeCN)][BF4]2 and [Ni(PtBu2NBn2)(dmpm)(MeCN)][BF4]2 (dmpm = 1,2-bis(dimethylylphosphino)methane) were prepared by the reaction of [Ni(PtBu2NBn2)(CH3CN)2][BF4]2 with the corresponding diphosphine ligands. [Ni(PtBu2NBn2)(dmpe)(MeCN)][BF4]2 readily undergoes P–C/C–H bond cleavage in nitromethane. In sharp contrast, [Ni(PtBu2NBn2)(dmpm)][BF4]2 is stabilized by dmpm, a diphosphine with small bite angle, and does not show P–C/C–H bond cleavage reactivity. Computational results show that for complexes bearing less bulky diphosphine ligands, such as dmpm, the barriers for the rate-determining transition states are in some examples higher than 30 kcal/mol with the M06 functional, higher than those for complexes bearing more rigid or more bulky ligands, consistent with experimental studies. The calculated barriers for the first transition state correlated with increased values of the dihedral angle formed by the two NiP2 planes.
中文翻译:

控制镍双(二膦)配合物中的P–C / C–H键裂解:反应范围,机理和计算
报道了杂合[Ni(P 2 N 2)(二膦)] [BF 4 ] 2配合物的合成以及这些配合物中P 2 N 2配体的P C和CH键的裂解。产物是五配位络合物,其中Ni–C和P–H键已形成,形成了包含Ni–CH═NR 2的环状部分。[Ni(P 2 N 2)(二膦)] [BF 4 ] 2的反应性配合物受二膦的刚性,取代基的空间效应和二膦配体的碳连接基团的长度影响。带有刚性主链的二膦配体(例如,dmpbz,1,2-双(二甲基膦基)苯)或芳香族取代基(例如,dppe,1,2-双(二苯基膦基)乙烷)与[Ni(P t Bu 2 N Bn 2)(CH 3 CN)2 ] [BF 4 ] 2给出PC / CH键的裂解产物。[Ni(P t Bu 2 N Bn 2)(dmpe)(MeCN)] [BF 4 ] 2和[Ni(P t Bu 2通过[Ni(P t Bu 2 N Bn 2)(CH 3 CN )的反应制备N Bn 2)(dmpm)(MeCN)] [BF 4 ] 2(dmpm = 1,2-双(二甲基基膦基)甲烷)。)2 ] [BF 4 ] 2与相应的二膦配体。[Ni(P t Bu 2 N Bn 2)(dmpe)(MeCN)] [BF 4 ] 2易在硝基甲烷中经历P–C / C–H键裂解。与之形成鲜明对比的是,[Ni(P t Bu 2 N Bn 2)(dmpm)] [BF 4] 2通过具有小咬合角的双膦dmpm稳定,并且不显示P–C / C–H键裂解反应性。计算结果表明,对于带有较小体积的二膦配体(例如dmpm)的配合物,在某些示例中,具有M06功能的决定速率过渡态的障碍高于30 kcal / mol,高于具有更大刚性或更高刚性的配合物庞大的配体,与实验研究一致。为第一过渡态计算的势垒与由两个NiP 2平面形成的二面角的增加值相关。
更新日期:2020-09-28
中文翻译:

控制镍双(二膦)配合物中的P–C / C–H键裂解:反应范围,机理和计算
报道了杂合[Ni(P 2 N 2)(二膦)] [BF 4 ] 2配合物的合成以及这些配合物中P 2 N 2配体的P C和CH键的裂解。产物是五配位络合物,其中Ni–C和P–H键已形成,形成了包含Ni–CH═NR 2的环状部分。[Ni(P 2 N 2)(二膦)] [BF 4 ] 2的反应性配合物受二膦的刚性,取代基的空间效应和二膦配体的碳连接基团的长度影响。带有刚性主链的二膦配体(例如,dmpbz,1,2-双(二甲基膦基)苯)或芳香族取代基(例如,dppe,1,2-双(二苯基膦基)乙烷)与[Ni(P t Bu 2 N Bn 2)(CH 3 CN)2 ] [BF 4 ] 2给出PC / CH键的裂解产物。[Ni(P t Bu 2 N Bn 2)(dmpe)(MeCN)] [BF 4 ] 2和[Ni(P t Bu 2通过[Ni(P t Bu 2 N Bn 2)(CH 3 CN )的反应制备N Bn 2)(dmpm)(MeCN)] [BF 4 ] 2(dmpm = 1,2-双(二甲基基膦基)甲烷)。)2 ] [BF 4 ] 2与相应的二膦配体。[Ni(P t Bu 2 N Bn 2)(dmpe)(MeCN)] [BF 4 ] 2易在硝基甲烷中经历P–C / C–H键裂解。与之形成鲜明对比的是,[Ni(P t Bu 2 N Bn 2)(dmpm)] [BF 4] 2通过具有小咬合角的双膦dmpm稳定,并且不显示P–C / C–H键裂解反应性。计算结果表明,对于带有较小体积的二膦配体(例如dmpm)的配合物,在某些示例中,具有M06功能的决定速率过渡态的障碍高于30 kcal / mol,高于具有更大刚性或更高刚性的配合物庞大的配体,与实验研究一致。为第一过渡态计算的势垒与由两个NiP 2平面形成的二面角的增加值相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号