当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrospun α-lactalbumin nanofibers for site-specific and fast-onset delivery of nicotine in the oral cavity: an in vitro, ex vivo and tissue spatial distribution study.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.molpharmaceut.0c00642 Kleopatra Kalouta 1, 2 , Mai Bay Stie 1, 2 , Christian Janfelt 1 , Ioannis S Chronakis 3 , Jette Jacobsen 1 , Hanne Mørck Nielsen 1, 2 , Vito Foderà 1, 2
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.molpharmaceut.0c00642 Kleopatra Kalouta 1, 2 , Mai Bay Stie 1, 2 , Christian Janfelt 1 , Ioannis S Chronakis 3 , Jette Jacobsen 1 , Hanne Mørck Nielsen 1, 2 , Vito Foderà 1, 2
Affiliation
|
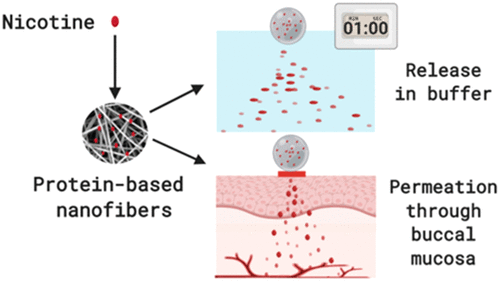
Nicotine replacement therapy (NRT) formulations for oromucosal administration induce a delayed rise in nicotine blood levels as opposed to the immediate nicotine increase obtained from cigarette smoking, this being a shortcoming of the therapy. Here, we demonstrate that α-lactalbumin/polyethylene oxide (ALA/PEO) electrospun nanofibers constitute an efficient oromucosal delivery system for fast-onset nicotine delivery of high relevance for acute dosing NRT applications. In vitro, nicotine-loaded nanofibers showed fast disintegration in water, with a weight loss up to 40% within minutes, and a faster nicotine release (26.1 ± 4.6% after 1 min of incubation) of the loaded nicotine compared to two relevant marketed NRT formulations with a comparable nicotine dose (i.e., 7.9 ± 5.1 and 2.2 ± 0.3% nicotine was released from a lozenge and a sublingual tablet, respectively). Model-fitting of the release data indicated that the release mechanism of nicotine from the hydrophilic nanofibers was possibly governed by more than one type of release phenomena. Remarkably, ex vivo studies using porcine buccal mucosa demonstrated a more efficient permeation of the nicotine released from the nanofibers [flux of 1.06 ± 0.22 nmol/(cm2·min)] compared to when dosing even a ten-fold concentrated nicotine solution [flux of 0.17 ± 0.14 nmol/(cm2·min)]. Moreover, matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI MS) imaging of ex vivo porcine buccal mucosa exposed to nicotine-loaded nanofibers clearly revealed higher amounts of nicotine throughout the epithelium, as well as in the lamina propria and submucosa of the tissue. Our findings suggest that nicotine-loaded ALA/PEO nanofibers have potential as a mucosal, fast-releasing, and biocompatible delivery system for nicotine, which can overcome the limitations of the currently marketed NRTs.
中文翻译:

电纺α-乳清蛋白纳米纤维用于口腔中尼古丁的位点特异性和快速递送:体外、离体和组织空间分布研究。
用于口腔粘膜给药的尼古丁替代疗法 (NRT) 制剂会导致血液中尼古丁水平的延迟升高,这与从吸烟中获得的尼古丁立即升高相反,这是该疗法的一个缺点。在这里,我们证明了 α-乳清蛋白/聚环氧乙烷 (ALA/PEO) 电纺纳米纤维构成了一种有效的口腔粘膜递送系统,用于与急性给药 NRT 应用高度相关的快速尼古丁递送。在体外,与两种相关的市售 NRT 相比,加载尼古丁的纳米纤维在水中显示出快速分解,几分钟内重量减轻高达 40%,并且加载尼古丁的尼古丁释放速度更快(孵育 1 分钟后为 26.1 ± 4.6%)具有相当尼古丁剂量的制剂(即、7.9 ± 5.1 和 2.2 ± 0.3% 的尼古丁分别从锭剂和舌下片中释放)。释放数据的模型拟合表明,亲水性纳米纤维中尼古丁的释放机制可能受不止一种释放现象的控制。值得注意的是,先体外后体内使用猪颊粘膜研究表明从纳米纤维[1.06±0.22纳摩尔/(厘米磁通释放的尼古丁的更有效的渗透2相比给药甚至当以10倍浓缩尼古丁溶液·分钟)] [磁通0.17 ± 0.14 nmol/(cm 2 ·min)]。此外,基质辅助激光解吸电离质谱成像 (MALDI MS)离体成像暴露于载有尼古丁的纳米纤维的猪颊粘膜清楚地显示整个上皮以及组织的固有层和粘膜下层中尼古丁含量更高。我们的研究结果表明,负载尼古丁的 ALA/PEO 纳米纤维具有作为尼古丁黏膜、快速释放和生物相容性输送系统的潜力,可以克服目前上市的 NRT 的局限性。
更新日期:2020-11-02
中文翻译:

电纺α-乳清蛋白纳米纤维用于口腔中尼古丁的位点特异性和快速递送:体外、离体和组织空间分布研究。
用于口腔粘膜给药的尼古丁替代疗法 (NRT) 制剂会导致血液中尼古丁水平的延迟升高,这与从吸烟中获得的尼古丁立即升高相反,这是该疗法的一个缺点。在这里,我们证明了 α-乳清蛋白/聚环氧乙烷 (ALA/PEO) 电纺纳米纤维构成了一种有效的口腔粘膜递送系统,用于与急性给药 NRT 应用高度相关的快速尼古丁递送。在体外,与两种相关的市售 NRT 相比,加载尼古丁的纳米纤维在水中显示出快速分解,几分钟内重量减轻高达 40%,并且加载尼古丁的尼古丁释放速度更快(孵育 1 分钟后为 26.1 ± 4.6%)具有相当尼古丁剂量的制剂(即、7.9 ± 5.1 和 2.2 ± 0.3% 的尼古丁分别从锭剂和舌下片中释放)。释放数据的模型拟合表明,亲水性纳米纤维中尼古丁的释放机制可能受不止一种释放现象的控制。值得注意的是,先体外后体内使用猪颊粘膜研究表明从纳米纤维[1.06±0.22纳摩尔/(厘米磁通释放的尼古丁的更有效的渗透2相比给药甚至当以10倍浓缩尼古丁溶液·分钟)] [磁通0.17 ± 0.14 nmol/(cm 2 ·min)]。此外,基质辅助激光解吸电离质谱成像 (MALDI MS)离体成像暴露于载有尼古丁的纳米纤维的猪颊粘膜清楚地显示整个上皮以及组织的固有层和粘膜下层中尼古丁含量更高。我们的研究结果表明,负载尼古丁的 ALA/PEO 纳米纤维具有作为尼古丁黏膜、快速释放和生物相容性输送系统的潜力,可以克服目前上市的 NRT 的局限性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号