当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring-in-Ring(s) Complexes Exhibiting Tunable Multicolor Photoluminescence
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/jacs.0c07745 Huang Wu 1 , Yu Wang 1 , Leighton O Jones 1 , Wenqi Liu 1 , Bo Song 1 , Yunpeng Cui 2 , Kang Cai 1 , Long Zhang 1 , Dengke Shen 1 , Xiao-Yang Chen 1 , Yang Jiao 1 , Charlotte L Stern 1 , Xiaopeng Li 3 , George C Schatz 1 , J Fraser Stoddart 1, 4, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/jacs.0c07745 Huang Wu 1 , Yu Wang 1 , Leighton O Jones 1 , Wenqi Liu 1 , Bo Song 1 , Yunpeng Cui 2 , Kang Cai 1 , Long Zhang 1 , Dengke Shen 1 , Xiao-Yang Chen 1 , Yang Jiao 1 , Charlotte L Stern 1 , Xiaopeng Li 3 , George C Schatz 1 , J Fraser Stoddart 1, 4, 5
Affiliation
|
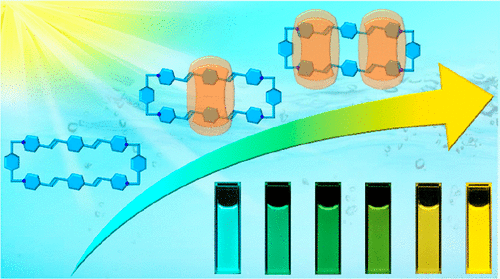
One ring threaded by two other rings to form a non-intertwined ternary ring-in-rings motif is a challenging task in noncovalent synthesis. Constructing multicolor photoluminescence systems with tunable properties is also a fundamental research goal, which can lead to applications in multidimensional biological imaging, visual displays, and encryption materials. Herein, we describe the design and synthesis of binary and ternary ring-in-ring(s) complexes, based on an extended tetracationic cyclophane and cucurbit[8]uril. The formation of these complexes is accompanied by tunable multicolor-fluorescence outputs. On mixing equimolar amounts of the cyclophane and cucurbit[8]uril, a 1:1 ring-in-ring complex is formed as a result of hydrophobic interactions associated with a favorable change in entropy. With the addition of another equivalent of cucurbit[8]uril, a 1:2 ring-in-rings complex is formed, facilitated by additional ion-dipole interactions involving the pyridinium units in the cyclophane and the carbonyl groups in cucurbit[8]uril. Because of the narrowing in the energy gaps of the cyclophane within the rigid hydrophobic cavities of cucurbit[8]urils, the binary and ternary ring-in-ring(s) complexes emit green and bright yellow fluorescence, respectively. A series of color-tunable emissions, such as skyblue, cyan, green, and yellow with increased fluorescence lifetimes, can be achieved by simply adding cucurbit[8]uril to an aqueous solution of the cyclophane. Notably, the smaller cyclobis(paraquat-p-phenylene), which contains the same p-xylylene linkers as the extended tetracationic cyclophane, does not form ring-in-ring(s) complexes with cucurbit[8]uril. The encapsulation of this extended tetracationic cyclophane by both one and two cucurbit[8]urils provides an incentive to design and synthesize more advanced supramolecular systems, as well as opening up a feasible approach toward achieving tunable multicolor photoluminescence with a single chromophore.
中文翻译:

显示可调多色光致发光的环中环配合物
一个环与其他两个环相连以形成非交织的三元环中基序是非共价合成中的一项具有挑战性的任务。构建具有可调特性的多色光致发光系统也是一个基本的研究目标,这可以导致在多维生物成像、视觉显示和加密材料方面的应用。在此,我们描述了基于扩展的四阳离子环芳和葫芦 [8] 脲的二元和三元环中配合物的设计和合成。这些复合物的形成伴随着可调的多色荧光输出。在混合等摩尔量的环芳烃和葫芦 [8] 脲时,由于与有利的熵变化相关的疏水相互作用,形成了 1:1 的环中环复合物。添加另一当量的葫芦 [8] 脲,形成 1:2 环中复合物,通过涉及环烷中的吡啶单元和葫芦 [8] 脲中的羰基的额外离子-偶极相互作用促进. 由于葫芦[8] 脲刚性疏水腔内环烷的能隙变窄,二元和三元环中配合物分别发出绿色和亮黄色荧光。通过简单地将葫芦[8]脲添加到环烷的水溶液中,可以实现一系列颜色可调的发射,例如天蓝色、青色、绿色和黄色,并增加荧光寿命。值得注意的是,较小的环双(百草枯-对亚苯基)含有与扩展的四阳离子环芳相同的对二甲苯连接基,不与葫芦 [8] 脲形成环中复合物。一个和两个葫芦 [8] 脲对这种扩展的四阳离子环芳的封装提供了设计和合成更先进的超分子系统的动力,并开辟了一种可行的方法来实现具有单个发色团的可调多色光致发光。
更新日期:2020-09-04
中文翻译:

显示可调多色光致发光的环中环配合物
一个环与其他两个环相连以形成非交织的三元环中基序是非共价合成中的一项具有挑战性的任务。构建具有可调特性的多色光致发光系统也是一个基本的研究目标,这可以导致在多维生物成像、视觉显示和加密材料方面的应用。在此,我们描述了基于扩展的四阳离子环芳和葫芦 [8] 脲的二元和三元环中配合物的设计和合成。这些复合物的形成伴随着可调的多色荧光输出。在混合等摩尔量的环芳烃和葫芦 [8] 脲时,由于与有利的熵变化相关的疏水相互作用,形成了 1:1 的环中环复合物。添加另一当量的葫芦 [8] 脲,形成 1:2 环中复合物,通过涉及环烷中的吡啶单元和葫芦 [8] 脲中的羰基的额外离子-偶极相互作用促进. 由于葫芦[8] 脲刚性疏水腔内环烷的能隙变窄,二元和三元环中配合物分别发出绿色和亮黄色荧光。通过简单地将葫芦[8]脲添加到环烷的水溶液中,可以实现一系列颜色可调的发射,例如天蓝色、青色、绿色和黄色,并增加荧光寿命。值得注意的是,较小的环双(百草枯-对亚苯基)含有与扩展的四阳离子环芳相同的对二甲苯连接基,不与葫芦 [8] 脲形成环中复合物。一个和两个葫芦 [8] 脲对这种扩展的四阳离子环芳的封装提供了设计和合成更先进的超分子系统的动力,并开辟了一种可行的方法来实现具有单个发色团的可调多色光致发光。









































 京公网安备 11010802027423号
京公网安备 11010802027423号