当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase Equilibria, Diffusivities, and Equation of State Modeling of HFC-32 and HFC-125 in Imidazolium-Based Ionic Liquids for the Separation of R-410A
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.iecr.0c02820 Ana Rita C. Morais 1 , Abby N. Harders 1, 2 , Kalin R. Baca 1, 2 , Greta M. Olsen 1, 2 , Bridgette J. Befort 3 , Alexander W. Dowling 3 , Edward J. Maginn 3 , Mark B. Shiflett 1, 2
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.iecr.0c02820 Ana Rita C. Morais 1 , Abby N. Harders 1, 2 , Kalin R. Baca 1, 2 , Greta M. Olsen 1, 2 , Bridgette J. Befort 3 , Alexander W. Dowling 3 , Edward J. Maginn 3 , Mark B. Shiflett 1, 2
Affiliation
|
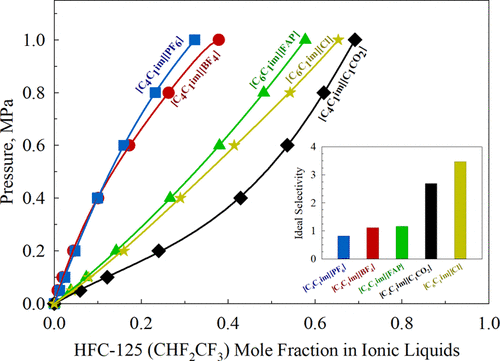
Growing concerns about the global warming potential of hydrofluorocarbons (HFCs) has led to increasing interest in developing technologies to effectively recover and recycle these refrigerants. Ionic liquids (ILs) have shown great potential to selectively separate azeotropic HFC gas mixtures, such as R-410A composed of HFC-32 (CH2F2) and HFC-125 (CHF2CF3), based on solubility differences between the refrigerant gases in the respective IL. Isothermal vapor–liquid equilibrium (VLE) data for HFC-32 and HFC-125 were measured in ILs containing fluorinated and nonfluorinated anions using a gravimetric microbalance at pressures ranging from 0.05 to 1.0 MPa and a temperature of 298.15 K. The van der Waals equation of state (EoS) model was applied to correlate the experimental solubility data of each HFC refrigerant/IL mixture. The solubility differences between HFC-32 and HFC-125 vary significantly depending on the choice of IL. The diffusion coefficients for both HFC refrigerants in each IL were calculated by fitting Fick’s law to time-dependent absorption data. HFC-32 has a higher diffusivity in most ILs tested because of its smaller molecular radius relative to HFC-125. Based on the calculated Henry’s law constants and the mass uptake for each system, [C6C1im][Cl] was found to have the highest selectivity difference for separating R-410A at 298.15 K.
中文翻译:

咪唑鎓离子液体中HFC-32和HFC-125的相平衡,扩散度和状态方程模型,用于R-410A的分离
人们越来越关注氢氟碳化合物(HFC)在全球变暖的潜力,这引起了人们对开发有效回收和再循环这些制冷剂的技术的兴趣。离子液体(IL)表现出巨大的潜力,可以选择性地分离共沸HFC气体混合物,例如由HFC-32(CH 2 F 2)和HFC-125(CHF 2 CF 3)组成的R-410A),基于各个IL中制冷剂气体之间的溶解度差异。使用重量微量天平,在0.05至1.0 MPa的压力和298.15 K的温度下,通过重量微量天平测量了HFC-32和HFC-125的等温气液平衡(VLE)数据。Van der Waals方程状态模型(EoS)用于关联每种HFC制冷剂/ IL混合物的实验溶解度数据。HFC-32和HFC-125之间的溶解度差异会根据IL的选择而有很大差异。通过将菲克定律与时间相关的吸收数据拟合,可以计算出每个离子液体中两种HFC制冷剂的扩散系数。HFC-32相对于HFC-125具有较小的分子半径,因此在大多数测试的IL中具有较高的扩散率。发现6 C 1 im] [Cl]在298.15 K分离R-410A具有最高的选择性差异。
更新日期:2020-10-07
中文翻译:

咪唑鎓离子液体中HFC-32和HFC-125的相平衡,扩散度和状态方程模型,用于R-410A的分离
人们越来越关注氢氟碳化合物(HFC)在全球变暖的潜力,这引起了人们对开发有效回收和再循环这些制冷剂的技术的兴趣。离子液体(IL)表现出巨大的潜力,可以选择性地分离共沸HFC气体混合物,例如由HFC-32(CH 2 F 2)和HFC-125(CHF 2 CF 3)组成的R-410A),基于各个IL中制冷剂气体之间的溶解度差异。使用重量微量天平,在0.05至1.0 MPa的压力和298.15 K的温度下,通过重量微量天平测量了HFC-32和HFC-125的等温气液平衡(VLE)数据。Van der Waals方程状态模型(EoS)用于关联每种HFC制冷剂/ IL混合物的实验溶解度数据。HFC-32和HFC-125之间的溶解度差异会根据IL的选择而有很大差异。通过将菲克定律与时间相关的吸收数据拟合,可以计算出每个离子液体中两种HFC制冷剂的扩散系数。HFC-32相对于HFC-125具有较小的分子半径,因此在大多数测试的IL中具有较高的扩散率。发现6 C 1 im] [Cl]在298.15 K分离R-410A具有最高的选择性差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号