当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coprecipitation of Phosphate and Silicate Affects Environmental Iron (Oxyhydr)Oxide Transformations: A Gel-Based Diffusive Sampler Approach.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.est.0c02352 Peter Kraal 1, 2 , Case M van Genuchten 1 , Wytze K Lenstra 1 , Thilo Behrends 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.est.0c02352 Peter Kraal 1, 2 , Case M van Genuchten 1 , Wytze K Lenstra 1 , Thilo Behrends 1
Affiliation
|
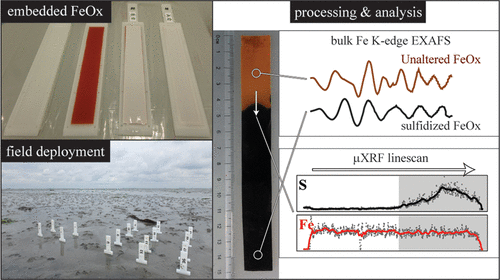
Sorption of nutrients such as phosphate (P) and silicate (Si) by ferric iron (oxyhydr)oxides (FeOx) modulates nutrient mobility and alters the structure and reactivity of the FeOx. We investigated the impact of these interactions on FeOx transformations using a novel approach with samplers containing synthetic FeOx embedded in diffusive hydrogels. The FeOx were prepared by Fe(III) hydrolysis and Fe(II) oxidation, in the absence and presence of P or Si. Coprecipitation of P or Si during synthesis altered the structure of Fe precipitates and, in the case of Fe(II) oxidation, lepidocrocite was (partly) substituted by poorly ordered FeOx. The pure and P- or Si-bearing FeOx were deployed in (i) freshwater sediment rich in dissolved Fe(II) and P and (ii) marine sediment with sulfidic pore water. Iron(II)-catalyzed crystallization of poorly ordered FeOx was negligible, likely due to surface passivation by adsorption of dissolved P. Reaction with dissolved sulfide was modulated by diffusion limitations and therefore the extent of sulfidation was the lowest for poorly ordered FeOx with high reactivity toward sulfide that created temporary, local sulfide depletion (Fh < Lp). We show that coprecipitation-induced changes in the FeOx structure affect coupled iron-nutrient cycling in aquatic ecosystems. The gel-based method enriches our geochemical toolbox by enabling detailed characterization of target phases under natural conditions.
中文翻译:

磷酸盐和硅酸盐的共沉淀影响环境铁(羟基)氧化物的转化:基于凝胶的扩散采样器方法。
铁(羟基氧化物)铁(FeOx)对诸如磷酸盐(P)和硅酸盐(Si)等营养物的吸附作用可调节营养物的迁移率并改变其结构和反应性。我们使用包含嵌入扩散水凝胶中的合成FeOx的采样器的新型方法,研究了这些相互作用对FeOx转化的影响。通过在不存在或不存在P或Si的条件下,通过Fe(III)水解和Fe(II)氧化来制备FeOx。合成过程中P或Si的共沉淀改变了Fe沉淀物的结构,并且在Fe(II)氧化的情况下,纤铁矿(部分)被不良有序的FeOx取代。纯的和含P或含Si的FeOx被部署在(i)富含溶解的Fe(II)和P的淡水沉积物中,以及(ii)具有硫化孔隙水的海洋沉积物中。铁(II)催化的有序FeOx的结晶微不足道,这很可能是由于溶解的P的吸附引起的表面钝化所致。与溶解的硫化物的反应受扩散限制的调节,因此对于反应性较差的FeOx,具有高反应性的硫化程度最低产生暂时的局部硫化物耗竭的硫化物(Fh <Lp)。我们表明,FeOx结构中的共沉淀诱导的变化会影响水生生态系统中耦合的铁养分循环。通过基于凝胶的方法,可以在自然条件下对目标相进行详细表征,从而丰富了我们的地球化学工具箱。与溶解的硫化物的反应受扩散限制的调节,因此,对于有序的FeOx而言,硫化程度最低,对硫化物的反应性高,会造成暂时的局部硫化物耗尽(Fh <Lp)。我们表明,FeOx结构中的共沉淀诱导的变化会影响水生生态系统中耦合的铁养分循环。通过基于凝胶的方法,可以在自然条件下对目标相进行详细表征,从而丰富了我们的地球化学工具箱。与溶解的硫化物的反应受扩散限制的调节,因此,对于有序的FeOx而言,硫化程度最低,对硫化物的反应性高,会造成暂时的局部硫化物耗尽(Fh <Lp)。我们表明,FeOx结构中的共沉淀诱导的变化会影响水生生态系统中耦合的铁养分循环。通过基于凝胶的方法,可以在自然条件下对目标相进行详细表征,从而丰富了我们的地球化学工具箱。
更新日期:2020-10-06
中文翻译:

磷酸盐和硅酸盐的共沉淀影响环境铁(羟基)氧化物的转化:基于凝胶的扩散采样器方法。
铁(羟基氧化物)铁(FeOx)对诸如磷酸盐(P)和硅酸盐(Si)等营养物的吸附作用可调节营养物的迁移率并改变其结构和反应性。我们使用包含嵌入扩散水凝胶中的合成FeOx的采样器的新型方法,研究了这些相互作用对FeOx转化的影响。通过在不存在或不存在P或Si的条件下,通过Fe(III)水解和Fe(II)氧化来制备FeOx。合成过程中P或Si的共沉淀改变了Fe沉淀物的结构,并且在Fe(II)氧化的情况下,纤铁矿(部分)被不良有序的FeOx取代。纯的和含P或含Si的FeOx被部署在(i)富含溶解的Fe(II)和P的淡水沉积物中,以及(ii)具有硫化孔隙水的海洋沉积物中。铁(II)催化的有序FeOx的结晶微不足道,这很可能是由于溶解的P的吸附引起的表面钝化所致。与溶解的硫化物的反应受扩散限制的调节,因此对于反应性较差的FeOx,具有高反应性的硫化程度最低产生暂时的局部硫化物耗竭的硫化物(Fh <Lp)。我们表明,FeOx结构中的共沉淀诱导的变化会影响水生生态系统中耦合的铁养分循环。通过基于凝胶的方法,可以在自然条件下对目标相进行详细表征,从而丰富了我们的地球化学工具箱。与溶解的硫化物的反应受扩散限制的调节,因此,对于有序的FeOx而言,硫化程度最低,对硫化物的反应性高,会造成暂时的局部硫化物耗尽(Fh <Lp)。我们表明,FeOx结构中的共沉淀诱导的变化会影响水生生态系统中耦合的铁养分循环。通过基于凝胶的方法,可以在自然条件下对目标相进行详细表征,从而丰富了我们的地球化学工具箱。与溶解的硫化物的反应受扩散限制的调节,因此,对于有序的FeOx而言,硫化程度最低,对硫化物的反应性高,会造成暂时的局部硫化物耗尽(Fh <Lp)。我们表明,FeOx结构中的共沉淀诱导的变化会影响水生生态系统中耦合的铁养分循环。通过基于凝胶的方法,可以在自然条件下对目标相进行详细表征,从而丰富了我们的地球化学工具箱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号