当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Biochemical Lanthanide-Encoding Approach Enables Quantitative Monitoring of the Bacterial Response to Vancomycin Treatment.
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.biochem.0c00614 Weitong Zhao 1 , Yong Liang 1 , Xiaowen Yan 1 , Limin Yang 1 , Qiuquan Wang 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.biochem.0c00614 Weitong Zhao 1 , Yong Liang 1 , Xiaowen Yan 1 , Limin Yang 1 , Qiuquan Wang 1
Affiliation
|
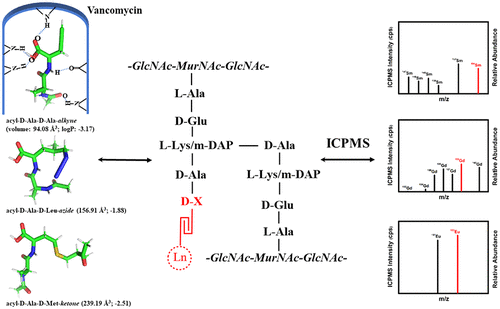
A pathogenic bacterium has its own mechanisms for not only pathogenic attack but also exogenous invasion defense, in which the bacterial cell wall is the front line of attack and defense. We developed a biochemical lanthanide-encoding approach to quantify the uncanonical d-amino acid (d-X) that was edited in a small proportion into the terminal acyl-d-Ala-d-X of nascent peptidoglycan UDP-MurNAc-pentapeptides in the bacterial cell wall. This approach overcomes the difficulties regarding quantification and accuracy issues encountered by the popular optical imaging and traditional high-performance liquid chromatography-based methods. Newly synthesized azide-d-Leu and ketone-d-Met were used together with alkynyl-d-Ala for their metabolic assembly and then bioorthogonally encoded by the correspondingly fabricated DBCO-DOTA-Gd, H2NO-DOTA-Eu, and azide-DOTA-Sm tags. This approach allows direct quantification of the d-X in situ in the cell wall using 158Gd, 153Eu, and 154Sm species-unspecific isotope dilution inductively coupled plasma mass spectrometry, avoiding any tedious and complex “cell-broken” pretreatment procedures that might induce racemization of the d-X. The obtained site-specific and accurate in situ information about the d-X enables quantitative monitoring of the bacterial response when Staphylococcus aureus meets vancomycin, showing that the amounts of azide-d-Leu and ketone-d-Met assembled are more important after determining the structure- and composition-dependent bacterial antibiotic resistance mechanisms. In addition, we found that the combined use of vancomycin and d-Ala restores the efficacy of vancomycin and might be a wise and simple way to combat vancomycin intermediate-resistant S. aureus.
中文翻译:

一种生化镧系元素编码方法能够对万古霉素治疗的细菌反应进行定量监测。
致病细菌不仅具有自身的机制,不仅可以用于致病性攻击,还可以用于外源性入侵防御,其中细菌细胞壁是攻击和防御的前线。我们开发了一种生化镧系元素的编码方法来量化非正交d -氨基甲酸(d这是在一小部分编辑到终端-X)酰基d -Ala- d新生肽聚糖在UDP-MurNAc-五肽的-X细菌细胞壁。这种方法克服了流行的光学成像和传统的基于高效液相色谱的方法所遇到的有关定量和准确性问题的难题。新合成的叠氮化物- ð -Leu和酮- d -Met用一起使用炔基- d -Ala为它们的代谢组件,然后bioorthogonally由相应编码制造DBCO -DOTA的Gd,ħ 2 NO -DOTA-Eu和叠氮化物-DOTA-SM的标记。这种方法允许使用158 Gd,153 Eu和154 Sm物种-非特异性同位素稀释电感耦合等离子体质谱法直接定量检测细胞壁中的d- X ,避免了任何繁琐而复杂的“细胞破碎”预处理程序可能会引起d的消旋-X。将所获得的位点特异性和准确围绕原位信息d -X使得当细菌响应的定量监测金黄色葡萄球菌满足万古霉素,显示出的量的叠氮化物- d -Leu和酮- ð -Met组装是决定后更重要依赖于结构和成分的细菌抗生素抗性机制。此外,我们发现万古霉素和d- Ala的联合使用可恢复万古霉素的功效,并且可能是对抗万古霉素中等耐药性金黄色葡萄球菌的明智而简单的方法。
更新日期:2020-09-29
中文翻译:

一种生化镧系元素编码方法能够对万古霉素治疗的细菌反应进行定量监测。
致病细菌不仅具有自身的机制,不仅可以用于致病性攻击,还可以用于外源性入侵防御,其中细菌细胞壁是攻击和防御的前线。我们开发了一种生化镧系元素的编码方法来量化非正交d -氨基甲酸(d这是在一小部分编辑到终端-X)酰基d -Ala- d新生肽聚糖在UDP-MurNAc-五肽的-X细菌细胞壁。这种方法克服了流行的光学成像和传统的基于高效液相色谱的方法所遇到的有关定量和准确性问题的难题。新合成的叠氮化物- ð -Leu和酮- d -Met用一起使用炔基- d -Ala为它们的代谢组件,然后bioorthogonally由相应编码制造DBCO -DOTA的Gd,ħ 2 NO -DOTA-Eu和叠氮化物-DOTA-SM的标记。这种方法允许使用158 Gd,153 Eu和154 Sm物种-非特异性同位素稀释电感耦合等离子体质谱法直接定量检测细胞壁中的d- X ,避免了任何繁琐而复杂的“细胞破碎”预处理程序可能会引起d的消旋-X。将所获得的位点特异性和准确围绕原位信息d -X使得当细菌响应的定量监测金黄色葡萄球菌满足万古霉素,显示出的量的叠氮化物- d -Leu和酮- ð -Met组装是决定后更重要依赖于结构和成分的细菌抗生素抗性机制。此外,我们发现万古霉素和d- Ala的联合使用可恢复万古霉素的功效,并且可能是对抗万古霉素中等耐药性金黄色葡萄球菌的明智而简单的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号