Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Topology of the U12–U6atac snRNA Complex of the Minor Spliceosome and Binding by NTC-Related Protein RBM22
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-04 , DOI: 10.1021/acsomega.0c01674 Joanna Ciavarella 1, 2 , William Perea 2 , Nancy L. Greenbaum 1, 2, 3
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-04 , DOI: 10.1021/acsomega.0c01674 Joanna Ciavarella 1, 2 , William Perea 2 , Nancy L. Greenbaum 1, 2, 3
Affiliation
|
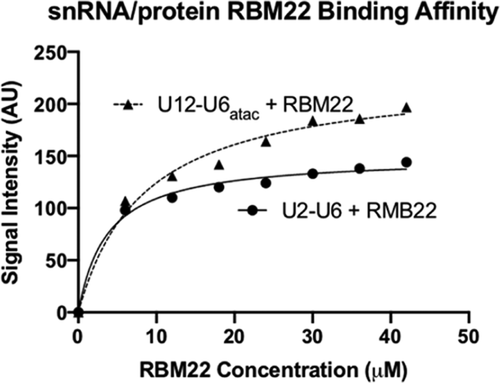
Splicing of precursor messenger RNA is catalyzed by the spliceosome, a dynamic ribonucleoprotein assembly including five small nuclear (sn)RNAs and >100 proteins. RNA components catalyze the two transesterification reactions, but proteins perform critical roles in assembly and rearrangement. The catalytic core comprises a paired complex of U2 and U6 snRNAs for the major form of the spliceosome and U12 and U6atac snRNAs for the minor variant (∼0.3% of all spliceosomes in higher eukaryotes); the latter shares key catalytic sequence elements and performs identical chemistry. Here we use solution NMR techniques to show that the U12–U6atac snRNA complex of both human and Arabidopsis maintain base-pairing patterns similar to those in the three-helix model of the U2–U6 snRNA complex that position key elements to form the spliceosome’s active site. However, in place of the stacked base pairs at the base of the U6 snRNA intramolecular stem loop and the central junction of the U2–U6 snRNA complex, we see altered geometry in the single-stranded hinge region opposing termini of the snRNAs to enable interaction between the key elements. We then use electrophoretic mobility shift assays and fluorescence assays to show that the protein RBM22, implicated in remodeling the human U2–U6 snRNA complex prior to catalysis, also binds the U12–U6atac snRNA complexes specifically and with similar affinity as to U2–U6 snRNA (a mean Kd for the two methods = 3.4 and 8.0 μM for U2–U6 and U12–U6atac snRNA complexes, respectively), suggesting that RBM22 performs the same role in both spliceosomes.
中文翻译:

小剪接体的U12–U6 atac snRNA复合体的拓扑以及与NTC相关蛋白RBM22的结合
前体信使RNA的剪接由剪接体催化,剪接体是一种动态的核糖核蛋白组装体,包括五个小核(sn)RNA和> 100个蛋白质。RNA成分催化两个酯交换反应,但蛋白质在组装和重排中起关键作用。催化核心包含一对主要形式的剪接体U2和U6 snRNA,以及次要变异体(高级真核生物中约0.3%的剪接体)的U12和U6 atac snRNA对。后者共享关键的催化序列元素,并执行相同的化学反应。在这里,我们使用溶液NMR技术来显示人和拟南芥的U12–U6 atac snRNA复合体维持碱基配对模式,类似于U2-U6 snRNA复合体的三螺旋模型中的模式,该模式将关键元件定位以形成剪接体的活性位点。然而,代替U6 snRNA分子内茎环和U2-U6 snRNA复合体中心连接处的堆叠碱基对,我们看到与snRNA末端相对的单链铰链区的几何形状发生了变化,从而实现了相互作用关键要素之间。然后,我们使用电泳迁移率变动分析和荧光分析法来证明蛋白质RBM22与催化之前的人U2-U6 snRNA复合物的重塑有关,它也特异性结合U12-U6 atac snRNA复合物,并且与U2-U6具有相似的亲和力snRNA(平均K d两种方法分别为U2-U6和U12-U6 atac snRNA复合体= 3.4和8.0μM),这表明RBM22在两个剪接体中都发挥相同的作用。
更新日期:2020-09-22
中文翻译:

小剪接体的U12–U6 atac snRNA复合体的拓扑以及与NTC相关蛋白RBM22的结合
前体信使RNA的剪接由剪接体催化,剪接体是一种动态的核糖核蛋白组装体,包括五个小核(sn)RNA和> 100个蛋白质。RNA成分催化两个酯交换反应,但蛋白质在组装和重排中起关键作用。催化核心包含一对主要形式的剪接体U2和U6 snRNA,以及次要变异体(高级真核生物中约0.3%的剪接体)的U12和U6 atac snRNA对。后者共享关键的催化序列元素,并执行相同的化学反应。在这里,我们使用溶液NMR技术来显示人和拟南芥的U12–U6 atac snRNA复合体维持碱基配对模式,类似于U2-U6 snRNA复合体的三螺旋模型中的模式,该模式将关键元件定位以形成剪接体的活性位点。然而,代替U6 snRNA分子内茎环和U2-U6 snRNA复合体中心连接处的堆叠碱基对,我们看到与snRNA末端相对的单链铰链区的几何形状发生了变化,从而实现了相互作用关键要素之间。然后,我们使用电泳迁移率变动分析和荧光分析法来证明蛋白质RBM22与催化之前的人U2-U6 snRNA复合物的重塑有关,它也特异性结合U12-U6 atac snRNA复合物,并且与U2-U6具有相似的亲和力snRNA(平均K d两种方法分别为U2-U6和U12-U6 atac snRNA复合体= 3.4和8.0μM),这表明RBM22在两个剪接体中都发挥相同的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号