当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Model-Based Design of Stable Electrolytes for Potassium Ion Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-09-04 , DOI: 10.1021/acsenergylett.0c01634 Jiao Zhang 1, 2 , Zhen Cao 3 , Lin Zhou 1, 2 , Geon-Tae Park 4 , Luigi Cavallo 3 , Limin Wang 1, 2 , Husam N. Alshareef 3 , Yang-Kook Sun 4 , Jun Ming 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-09-04 , DOI: 10.1021/acsenergylett.0c01634 Jiao Zhang 1, 2 , Zhen Cao 3 , Lin Zhou 1, 2 , Geon-Tae Park 4 , Luigi Cavallo 3 , Limin Wang 1, 2 , Husam N. Alshareef 3 , Yang-Kook Sun 4 , Jun Ming 1, 2
Affiliation
|
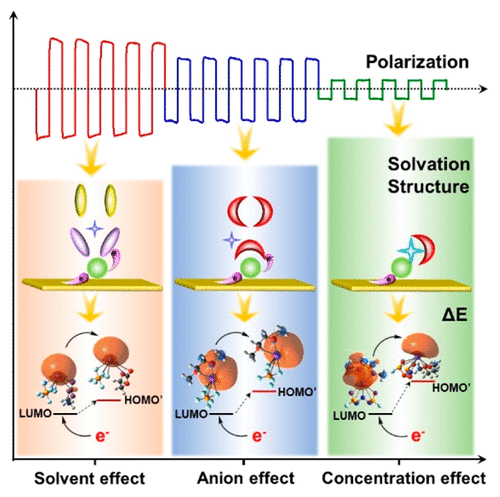
Rechargeable potassium (ion) batteries (KIBs) are an emerging energy storage system with many potential advantages over existing battery systems (e.g., Li-ion, Na-ion battery). However, the electrolyte design for KIBs remains challenging because of serious electrolyte decomposition. Particularly, this problem is aggravated when using metal potassium anodes. Herein, we report that the cation-solvent (i.e., K+-solvent) solvation structure, which is determined by the electrolyte composition, plays a dominant role in the failure of KIBs. We present an analysis of the reaction pathway to understand the behavior of the cation-solvent structure at the surface of metal electrodes (e.g., metal plating or M+-solvent decomposition). The electronegativity change of cation-solvent structure was studied and correlated to the stability of the electrolytes. We find that the electrolyte decomposition can be induced when the K+-solvent structure accepts one electron from the electrode; however, this process can be suppressed by tuning the electronegativity through varying the solvent chemistry, anion type, and salt concentrations. Our results explain the high stability of existing high-concentration electrolytes and present a general guideline to design stable electrolytes for KIBs. This approach can pave the way for the realization of high-performance K-ion batteries.
中文翻译:

基于模型的钾离子电池稳定电解质设计
可充电钾(离子)电池(KIB)是一种新兴的储能系统,与现有的电池系统(例如,锂离子,钠离子电池)相比,具有许多潜在的优势。然而,由于严重的电解质分解,用于KIB的电解质设计仍然具有挑战性。特别地,当使用金属钾阳极时,该问题加剧。在此,我们报道了由电解质组成决定的阳离子-溶剂(即K + -溶剂)溶剂化结构在KIBs的失效中起着主导作用。我们对反应路径进行了分析,以了解金属电极表面(例如,金属镀层或M +-溶剂分解)。研究了阳离子-溶剂结构的电负性变化,并将其与电解质的稳定性相关。我们发现,当K +-溶剂结构从电极接受一个电子时,可以诱导电解质分解。但是,可以通过改变溶剂的化学性质,阴离子类型和盐浓度来调节电负性,从而抑制这一过程。我们的结果说明了现有高浓度电解质的高稳定性,并为设计用于KIB的稳定电解质提供了一般指导。这种方法可以为高性能K离子电池的实现铺平道路。
更新日期:2020-10-11
中文翻译:

基于模型的钾离子电池稳定电解质设计
可充电钾(离子)电池(KIB)是一种新兴的储能系统,与现有的电池系统(例如,锂离子,钠离子电池)相比,具有许多潜在的优势。然而,由于严重的电解质分解,用于KIB的电解质设计仍然具有挑战性。特别地,当使用金属钾阳极时,该问题加剧。在此,我们报道了由电解质组成决定的阳离子-溶剂(即K + -溶剂)溶剂化结构在KIBs的失效中起着主导作用。我们对反应路径进行了分析,以了解金属电极表面(例如,金属镀层或M +-溶剂分解)。研究了阳离子-溶剂结构的电负性变化,并将其与电解质的稳定性相关。我们发现,当K +-溶剂结构从电极接受一个电子时,可以诱导电解质分解。但是,可以通过改变溶剂的化学性质,阴离子类型和盐浓度来调节电负性,从而抑制这一过程。我们的结果说明了现有高浓度电解质的高稳定性,并为设计用于KIB的稳定电解质提供了一般指导。这种方法可以为高性能K离子电池的实现铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号