当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sensing Traction Force on the Matrix Induces Cell–Cell Distant Mechanical Communications for Self-Assembly
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-09-03 , DOI: 10.1021/acsbiomaterials.0c01035 Mingxing Ouyang 1 , Zhili Qian 1 , Bing Bu 1 , Yang Jin 1 , Jiajia Wang 1 , Yiming Zhu 1 , Lei Liu 1 , Yan Pan 1 , Linhong Deng 1
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-09-03 , DOI: 10.1021/acsbiomaterials.0c01035 Mingxing Ouyang 1 , Zhili Qian 1 , Bing Bu 1 , Yang Jin 1 , Jiajia Wang 1 , Yiming Zhu 1 , Lei Liu 1 , Yan Pan 1 , Linhong Deng 1
Affiliation
|
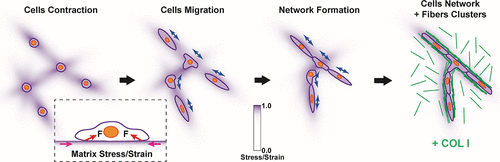
The long-range biomechanical force propagating across a large scale may reserve the capability to trigger coordinative responses within cell population such as during angiogenesis, epithelial tubulogenesis, and cancer metastasis. How cells communicate in a distant manner within the group for self-assembly remains largely unknown. Here, we found that airway smooth muscle cells (ASMCs) rapidly self-assembled into a well-constructed network on 3D Matrigel containing type I collagen (COL), which relied on long-range biomechanical force across the matrix to direct cell–cell distant interactions. Similar results happened by HUVEC cells to mimic angiogenesis. Interestingly, single ASMCs initiated multiple extended protrusions precisely pointing to neighboring cells in distance (100–300 μm away or 5–10 folds of the diameter of a round single cell), depending on traction force sensing. Individual ASMCs mechanosensed each other to move directionally on both nonfibrous Matrigel only and Matrigel containing fibrous COL but lost mutual sensing on the cross-linked gel or coated glass due to no long-range force transmission. The bead tracking assay demonstrated distant transmission of traction force (up to 400 μm) during the matrix deformation, and finite element method modeling confirmed the consistency between maximum strain distribution on the matrix and cell directional movements in experiments. Furthermore, ASMCs recruited COL from the hydrogel to build a fibrous network to mechanically stabilize the cell network. Our results revealed principally that cells can sense traction force transmitted through the matrix to initiate cell–cell distant mechanical communications, resulting in cell directional migration and coordinated cell and COL self-assembly with active matrix remodeling. As an interesting phenomenon, cells seem to be able to “make a phone call” via long-range biomechanics, which implicates physiological importance such as for tissue pattern formation.
中文翻译:

基质上的传感牵引力可诱导细胞间自组装的远距离机械通讯
大规模传播的远距离生物力学力可能保留了触发细胞群体内协调反应的能力,例如在血管生成,上皮小管生成和癌症转移过程中。细胞如何在组内以远距离方式通信以进行自组装仍然很大程度上未知。在这里,我们发现气道平滑肌细胞(ASMC)在含有I型胶原(COL)的3D Matrigel上迅速自组装成结构良好的网络,该网络依靠穿过基质的长期生物力学力来引导细胞与细胞之间的距离互动。HUVEC细胞模拟血管生成也发生了类似的结果。有趣的是,单个ASMC会引发多个延伸的突起,精确地指向远处的相邻细胞(相距100-300μm或圆形单细胞直径的5-10倍),取决于牵引力感应。各个ASMC相互机械感应,以便仅在非纤维基质胶和含纤维COL的基质胶上定向移动,但由于没有长程力传递,因此在交联凝胶或涂层玻璃上失去了相互感应。磁珠跟踪试验表明,在基质变形过程中,牵引力会远距离传递(最大400μm),而有限元方法建模证实了实验中基质上最大应变分布与细胞定向运动之间的一致性。此外,ASMC从水凝胶中吸收了COL,以建立纤维网络来机械稳定细胞网络。我们的结果主要表明,细胞可以感知通过基质传递的牵引力,从而启动细胞与细胞之间的远距离机械通信,导致细胞定向迁移,并通过主动矩阵重构实现协调的细胞和COL自组装。作为一个有趣的现象,细胞似乎能够通过远程生物力学“打个电话”,这暗示了生理重要性,例如对于组织模式的形成。
更新日期:2020-10-12
中文翻译:

基质上的传感牵引力可诱导细胞间自组装的远距离机械通讯
大规模传播的远距离生物力学力可能保留了触发细胞群体内协调反应的能力,例如在血管生成,上皮小管生成和癌症转移过程中。细胞如何在组内以远距离方式通信以进行自组装仍然很大程度上未知。在这里,我们发现气道平滑肌细胞(ASMC)在含有I型胶原(COL)的3D Matrigel上迅速自组装成结构良好的网络,该网络依靠穿过基质的长期生物力学力来引导细胞与细胞之间的距离互动。HUVEC细胞模拟血管生成也发生了类似的结果。有趣的是,单个ASMC会引发多个延伸的突起,精确地指向远处的相邻细胞(相距100-300μm或圆形单细胞直径的5-10倍),取决于牵引力感应。各个ASMC相互机械感应,以便仅在非纤维基质胶和含纤维COL的基质胶上定向移动,但由于没有长程力传递,因此在交联凝胶或涂层玻璃上失去了相互感应。磁珠跟踪试验表明,在基质变形过程中,牵引力会远距离传递(最大400μm),而有限元方法建模证实了实验中基质上最大应变分布与细胞定向运动之间的一致性。此外,ASMC从水凝胶中吸收了COL,以建立纤维网络来机械稳定细胞网络。我们的结果主要表明,细胞可以感知通过基质传递的牵引力,从而启动细胞与细胞之间的远距离机械通信,导致细胞定向迁移,并通过主动矩阵重构实现协调的细胞和COL自组装。作为一个有趣的现象,细胞似乎能够通过远程生物力学“打个电话”,这暗示了生理重要性,例如对于组织模式的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号