当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Launching Werner Complexes into the Modern Era of Catalytic Enantioselective Organic Synthesis.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.accounts.0c00410 Aaron R Wegener 1 , Connor Q Kabes 1 , John A Gladysz 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/acs.accounts.0c00410 Aaron R Wegener 1 , Connor Q Kabes 1 , John A Gladysz 1
Affiliation
|
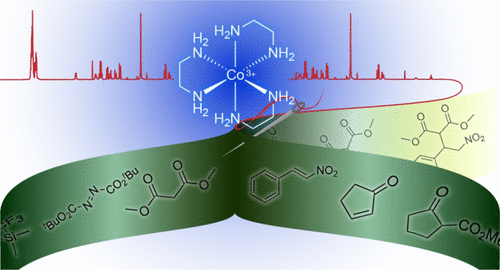
Reactions catalyzed by transition metal complexes almost always entail binding of one or more reactants to the metal center, and nearly every corner of the “chiral pool” has been picked over in efforts to develop enantioselective catalysts. As reported by Alfred Werner in 1911–1912, salts of the formally D3-symmetric [Co(en)3]3+ trication (en = ethylenediamine) were among the first chiral inorganic compounds to be resolved into enantiomers. These air- and water-stable complexes are substitution-inert, so for 100 years they languished without application in organic synthesis. We then showed that when they are rendered soluble in organic media by lipophilic anions such as fluorinated tetraarylborates BArf–, they become potent catalysts for a variety of carbon–carbon and carbon–heteroatom bond forming reactions.
中文翻译:

将Werner配合物推向催化对映选择性有机合成的现代时代。
过渡金属络合物催化的反应几乎总是需要一种或多种反应物与金属中心结合,并且在开发对映选择性催化剂的努力中,几乎已经吸收了“手性池”的每个角落。正如阿尔弗雷德·沃纳(Alfred Werner)在1911年至1912年所报告的那样,形式上为D 3对称的[Co(en)3 ] 3+三阳离子(en =乙二胺)的盐是最早被拆分为对映体的手性无机化合物。这些对空气和水稳定的络合物是惰性取代的,因此100多年来,它们在没有应用于有机合成的过程中一直受到限制。然后,我们证明了当它们被亲脂性阴离子(如氟化四芳基硼酸酯BAr f –,它们成为各种碳-碳和碳-杂原子键形成反应的有效催化剂。
更新日期:2020-10-21
中文翻译:

将Werner配合物推向催化对映选择性有机合成的现代时代。
过渡金属络合物催化的反应几乎总是需要一种或多种反应物与金属中心结合,并且在开发对映选择性催化剂的努力中,几乎已经吸收了“手性池”的每个角落。正如阿尔弗雷德·沃纳(Alfred Werner)在1911年至1912年所报告的那样,形式上为D 3对称的[Co(en)3 ] 3+三阳离子(en =乙二胺)的盐是最早被拆分为对映体的手性无机化合物。这些对空气和水稳定的络合物是惰性取代的,因此100多年来,它们在没有应用于有机合成的过程中一直受到限制。然后,我们证明了当它们被亲脂性阴离子(如氟化四芳基硼酸酯BAr f –,它们成为各种碳-碳和碳-杂原子键形成反应的有效催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号