当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical research on the direct carboxylation of benzene with CO2 catalyzed by different carbene‐CuOH compounds
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-09-04 , DOI: 10.1002/poc.4137 Cong Liu 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-09-04 , DOI: 10.1002/poc.4137 Cong Liu 1
Affiliation
|
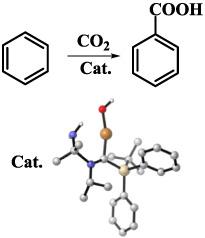
The direct carboxylation between the benzene C–H and CO2 is a long‐standing challenge in catalysis. Based on the known experimental process for the direct carboxylation of fluorobenzene, herein, a theoretical design of efficient carbene‐copper catalysts for the direct carboxylation of benzene was reported. It was found that the reaction included two steps: C–H bond breaking and CO2 insertion into Cu‐phenyl. C–H breaking is the rate‐determining step. To find out a more effective catalyst, totally, 16 carbene‐copper compounds with different electronic and steric structures were investigated. The energy barriers for the C–H bond breaking range from 104.2 to 153.7 kJ/mol. Two effective methods are proposed for improving the catalytic reactivity: reducing the electronically active substituent on carbene and introducing interaction to stabilize the transition state (TS). Based on these findings, adopting a well‐known (amino)(silyl)carbene modified by a –NH2 group gives the most effective catalyst for the direct carboxylation of benzene. Using –NH2 group can introduce two strong interactions (OOH···HNH2 and NNH2···Cu) during the formation of TS, which can greatly stabilize the structure of TS and dramatically reduce the energy barrier. The catalytic reactivity of the most effective carbene‐copper catalyst is comparable with the noble metal‐based ruthenium pincer carboxylate complexes.
中文翻译:

不同碳烯-CuOH化合物催化苯与CO2直接羧化的理论研究
苯CH和CO 2之间的直接羧化是催化领域的长期挑战。本文基于已知的直接将氟苯羧化的实验方法,报道了用于苯直接羧化的高效卡宾-铜催化剂的理论设计。发现该反应包括两个步骤:CH键断裂和CO 2插入铜苯基 C–H中断是确定速率的步骤。为了找到更有效的催化剂,总共研究了16种具有不同电子和空间结构的卡宾-铜化合物。C–H键断裂的能垒范围为104.2至153.7 kJ / mol。提出了两种有效的方法来改善催化反应性:还原卡宾上的电子活性取代基和引入相互作用以稳定过渡态(TS)。基于这些发现,采用由-NH 2基团修饰的众所周知的(氨基)(甲硅烷基)卡宾是最有效的苯直接羧化催化剂。使用–NH 2基团可以引入两个强相互作用(O OH ···H NH2和N在TS形成过程中产生NH2 ···Cu),可以极大地稳定TS的结构并显着降低能垒。最有效的卡宾-铜催化剂的催化反应性可与贵金属基钌夹钳羧酸盐络合物媲美。
更新日期:2020-09-04
中文翻译:

不同碳烯-CuOH化合物催化苯与CO2直接羧化的理论研究
苯CH和CO 2之间的直接羧化是催化领域的长期挑战。本文基于已知的直接将氟苯羧化的实验方法,报道了用于苯直接羧化的高效卡宾-铜催化剂的理论设计。发现该反应包括两个步骤:CH键断裂和CO 2插入铜苯基 C–H中断是确定速率的步骤。为了找到更有效的催化剂,总共研究了16种具有不同电子和空间结构的卡宾-铜化合物。C–H键断裂的能垒范围为104.2至153.7 kJ / mol。提出了两种有效的方法来改善催化反应性:还原卡宾上的电子活性取代基和引入相互作用以稳定过渡态(TS)。基于这些发现,采用由-NH 2基团修饰的众所周知的(氨基)(甲硅烷基)卡宾是最有效的苯直接羧化催化剂。使用–NH 2基团可以引入两个强相互作用(O OH ···H NH2和N在TS形成过程中产生NH2 ···Cu),可以极大地稳定TS的结构并显着降低能垒。最有效的卡宾-铜催化剂的催化反应性可与贵金属基钌夹钳羧酸盐络合物媲美。











































 京公网安备 11010802027423号
京公网安备 11010802027423号