当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst and Additive‐Free Direct Amidation/Halogenation of Tertiary Arylamines with N‐haloimide/amides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-03 , DOI: 10.1002/adsc.202000796 Xiu‐Juan Xu 1 , Adila Amuti 1 , Abudureheman Wusiman 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-03 , DOI: 10.1002/adsc.202000796 Xiu‐Juan Xu 1 , Adila Amuti 1 , Abudureheman Wusiman 1, 2
Affiliation
|
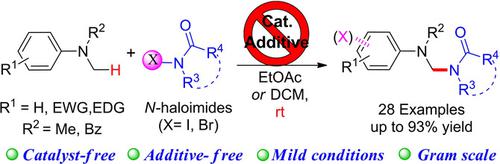
An approach has been developed for the amidation (halogenation) of tertiary arylamines by electrophilic activation using N‐haloimide/amides. Several control experiments have been performed, and the coupling reaction outcomes indicated that the N‐haloimide/amide brings three major functions, including electrophilic activation, aromatic halogenation and nucleophilic nitrogen sources. This cascade reaction features simple manipulation, requires no additional catalyst, oxidant or additives, and is performed under mild conditions.
中文翻译:

叔芳胺与N-卤代酰亚胺/酰胺的催化剂和无添加剂的直接酰胺化/卤化反应
已开发出一种方法,可使用N卤代酰亚胺/酰胺通过亲电活化来对叔芳基胺进行酰胺化(卤化)。已经进行了一些控制实验,偶联反应结果表明,N-卤代酰亚胺/酰胺具有三个主要功能,包括亲电活化,芳族卤化和亲核氮源。该级联反应具有操作简单,不需要额外的催化剂,氧化剂或添加剂的特点,并且在温和条件下进行。
更新日期:2020-09-03
中文翻译:

叔芳胺与N-卤代酰亚胺/酰胺的催化剂和无添加剂的直接酰胺化/卤化反应
已开发出一种方法,可使用N卤代酰亚胺/酰胺通过亲电活化来对叔芳基胺进行酰胺化(卤化)。已经进行了一些控制实验,偶联反应结果表明,N-卤代酰亚胺/酰胺具有三个主要功能,包括亲电活化,芳族卤化和亲核氮源。该级联反应具有操作简单,不需要额外的催化剂,氧化剂或添加剂的特点,并且在温和条件下进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号