当前位置:
X-MOL 学术
›
J. Inherit. Metab. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of triheptanoin (UX007) in patients with long‐chain fatty acid oxidation disorders: Results from an open‐label, long‐term extension study
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-09-04 , DOI: 10.1002/jimd.12313 Jerry Vockley 1 , Barbara Burton 2 , Gerard Berry 3 , Nicola Longo 4 , John Phillips 5 , Amarilis Sanchez-Valle 6 , Kimberly Chapman 7 , Pranoot Tanpaiboon 7 , Stephanie Grunewald 8 , Elaine Murphy 9 , Xiaoxiao Lu 10 , Jason Cataldo 10
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-09-04 , DOI: 10.1002/jimd.12313 Jerry Vockley 1 , Barbara Burton 2 , Gerard Berry 3 , Nicola Longo 4 , John Phillips 5 , Amarilis Sanchez-Valle 6 , Kimberly Chapman 7 , Pranoot Tanpaiboon 7 , Stephanie Grunewald 8 , Elaine Murphy 9 , Xiaoxiao Lu 10 , Jason Cataldo 10
Affiliation
|
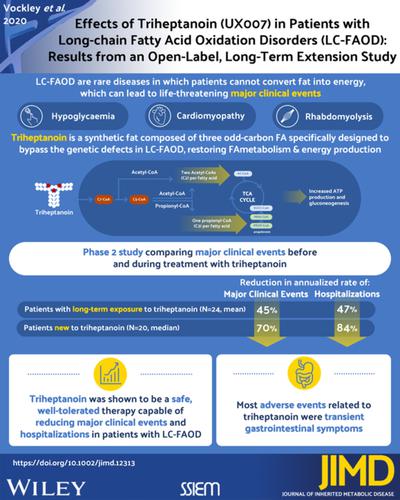
Long‐chain fatty acid oxidation disorders (LC‐FAOD) are autosomal recessive conditions that impair conversion of long‐chain fatty acids into energy, leading to significant clinical symptoms. Triheptanoin is a highly purified, 7‐carbon chain triglyceride approved in the United States as a source of calories and fatty acids for treatment of pediatric and adult patients with molecularly confirmed LC‐FAOD. CL202 is an open‐label, long‐term extension study evaluating triheptanoin (Dojolvi) safety and efficacy in patients with LC‐FAOD. Patients rolled over from the CL201 triheptanoin clinical trial (rollover); were triheptanoin‐naïve (naïve); or had participated in investigator‐sponsored trials/expanded access programs (IST/other). Results focus on rollover and naïve groups, as pretreatment data allow comparison. Primary outcomes were annual rate and duration of major clinical events (MCEs; rhabdomyolysis, hypoglycemia, and cardiomyopathy events). Seventy‐five patients were enrolled (24 rollover, 20 naïve, 31 IST/other). Mean study duration was 23.0 months for rollover, 15.7 months for naïve, and 34.7 months for IST/other. In the rollover group, mean annualized MCE rate decreased from 1.76 events/year pre‐triheptanoin to 0.96 events/year with triheptanoin (P = .0319). Median MCE duration was reduced by 66%. In the naïve group, median annualized MCE rate decreased from 2.33 events/year pre‐triheptanoin to 0.71 events/year with triheptanoin (P = .1072). Median MCE duration was reduced by 80%. The most common related adverse events (AEs) were diarrhea, abdominal pain/discomfort, and vomiting, most mild to moderate. Three patients had serious AEs (diverticulitis, ileus, rhabdomyolysis) possibly related to drug; all resolved. Two patients had AEs leading to death; neither drug related. Triheptanoin reduced rate and duration of MCEs. Safety was consistent with previous observations.
中文翻译:

三庚酸甘油酯 (UX007) 对长链脂肪酸氧化障碍患者的影响:一项开放标签、长期扩展研究的结果
长链脂肪酸氧化障碍 (LC-FAOD) 是常染色体隐性遗传病,可损害长链脂肪酸向能量的转化,导致显着的临床症状。三庚酸甘油酯是一种高度纯化的 7 碳链甘油三酯,在美国被批准作为卡路里和脂肪酸的来源,用于治疗分子确认 LC-FAOD 的儿童和成人患者。CL202 是一项开放标签的长期扩展研究,用于评估三庚酸甘油酯 (Dojolvi) 在 LC-FAOD 患者中的安全性和有效性。从 CL201 三庚酸甘油酯临床试验(rollover)转出的患者;未使用三庚酸甘油酯(未使用);或曾参与过研究者发起的试验/扩大访问计划(IST/其他)。结果集中在翻转组和初始组,因为预处理数据允许进行比较。主要结局是主要临床事件(MCE;横纹肌溶解、低血糖和心肌病事件)的年发生率和持续时间。招募了 75 名患者(24 名翻身,20 名初治,31 名 IST/其他)。轮转的平均研究持续时间为 23.0 个月,naïve 为 15.7 个月,IST/other 为 34.7 个月。在翻转组中,平均年化 MCE 率从使用三庚酸前的 1.76 事件/年下降到使用三庚酸的 0.96 事件/年。P = .0319)。MCE 持续时间中位数减少了 66%。在初治组中,年化 MCE 率中位数从使用三庚酸甘油酯前的 2.33 次事件/年下降到使用三庚酸甘油酯时的 0.71 次事件/年 ( P = .1072)。MCE 持续时间中位数减少了 80%。最常见的相关不良事件 (AE) 是腹泻、腹痛/不适和呕吐,大多数为轻度至中度。3 名患者出现可能与药物有关的严重 AE(憩室炎、肠梗阻、横纹肌溶解症);都解决了。两名患者有导致死亡的 AE;与药物无关。三庚酸甘油酯降低了 MCE 的发生率和持续时间。安全性与之前的观察结果一致。
更新日期:2020-09-04
中文翻译:

三庚酸甘油酯 (UX007) 对长链脂肪酸氧化障碍患者的影响:一项开放标签、长期扩展研究的结果
长链脂肪酸氧化障碍 (LC-FAOD) 是常染色体隐性遗传病,可损害长链脂肪酸向能量的转化,导致显着的临床症状。三庚酸甘油酯是一种高度纯化的 7 碳链甘油三酯,在美国被批准作为卡路里和脂肪酸的来源,用于治疗分子确认 LC-FAOD 的儿童和成人患者。CL202 是一项开放标签的长期扩展研究,用于评估三庚酸甘油酯 (Dojolvi) 在 LC-FAOD 患者中的安全性和有效性。从 CL201 三庚酸甘油酯临床试验(rollover)转出的患者;未使用三庚酸甘油酯(未使用);或曾参与过研究者发起的试验/扩大访问计划(IST/其他)。结果集中在翻转组和初始组,因为预处理数据允许进行比较。主要结局是主要临床事件(MCE;横纹肌溶解、低血糖和心肌病事件)的年发生率和持续时间。招募了 75 名患者(24 名翻身,20 名初治,31 名 IST/其他)。轮转的平均研究持续时间为 23.0 个月,naïve 为 15.7 个月,IST/other 为 34.7 个月。在翻转组中,平均年化 MCE 率从使用三庚酸前的 1.76 事件/年下降到使用三庚酸的 0.96 事件/年。P = .0319)。MCE 持续时间中位数减少了 66%。在初治组中,年化 MCE 率中位数从使用三庚酸甘油酯前的 2.33 次事件/年下降到使用三庚酸甘油酯时的 0.71 次事件/年 ( P = .1072)。MCE 持续时间中位数减少了 80%。最常见的相关不良事件 (AE) 是腹泻、腹痛/不适和呕吐,大多数为轻度至中度。3 名患者出现可能与药物有关的严重 AE(憩室炎、肠梗阻、横纹肌溶解症);都解决了。两名患者有导致死亡的 AE;与药物无关。三庚酸甘油酯降低了 MCE 的发生率和持续时间。安全性与之前的观察结果一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号