当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Study of Donor–Acceptor Bonds on the N‐Coordinated Sn/Pb(II) Atoms in peri‐Substituted Naphthalenes: Evidence of Pb→B Interaction
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-09-04 , DOI: 10.1002/ejic.202000696 Michal Aman 1 , Libor Dostál 1 , Tomáš Mikysek 2 , Zdenka Růžičková 1 , Stefan Mebs 3 , Jens Beckmann 4 , Roman Jambor 1
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-09-04 , DOI: 10.1002/ejic.202000696 Michal Aman 1 , Libor Dostál 1 , Tomáš Mikysek 2 , Zdenka Růžičková 1 , Stefan Mebs 3 , Jens Beckmann 4 , Roman Jambor 1
Affiliation
|
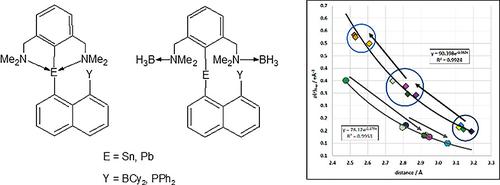
Peri‐substituted naphthalene derivatives, namely, N‐coordinated plumbylenes 1‐PPh2‐8‐PbL‐C10H6 (4) and 1‐BCy2‐8‐PbL‐C10H6 (5) (L = 2,6‐(Me2NCH2)2C6H3) were prepared. Compounds 4 and 5 allowed studying interactions between the Pb atoms with the either Lewis acidic (BCy2) or Lewis basic sites (PPh2). Addition of an external Lewis acid (BH3·SMe2) to 5 provided the complex 1‐BCy2‐8‐[(BH3)2L]Pb‐C10H6 (7), in which both NMe2 groups of the ligand L were coordinated by BH3. This reaction has also been used for the synthesis of a tin analogue 1‐BCy2‐8‐[(BH3)2L]Sn‐C10H6 (6). New complexes 4–7 were also compared with the previously known, tin(II) analogues 1‐PPh2‐8‐SnL‐C10H6 (1), 1‐PPh2‐8‐[(BH3)2L]Sn‐C10H6 (2), 1‐BCy2‐8‐SnL‐C10H6 (3). This set of compounds allowed us to study influence of the P→E and E→B peri‐contacts upon the redox potentials E1/2 (ox1) by the cyclic voltammetry. As the result, the selective oxidation of E(II) atom was also studied and the new organotin(IV) sulfide [1‐PPh2‐8‐LSn(C10H6)(µ‐S)]2 (9) was prepared, the product of exclusive oxidation of the tin(II) atom in 1. Beside this, the evaluation of the P→E and E→B peri‐contacts in the neutral molecules, the first and second ionization energies (IE) were calculated. For this reason, electronic properties, full structural optimizations were conducted with density functional theory (DFT) for all possible combinations of metal atom (Sn vs. Pb), Lewis acid (N→Sn/Pb vs. N(BH3)Sn/Pb), peri‐substituents (BCy2 vs. PPh2), and for three molecular charges (0 vs. 1+ vs. 2+) on the B3PW91/6‐311+g(2df,p) level of theory.
中文翻译:

取代萘中N-配位的Sn / Pb(II)原子上供体-受体键的研究:Pb→B相互作用的证据
围取代萘衍生物,即,Ñ配位的plumbylenes 1-PPH 2 -8-PBL-C 10 H ^ 6(4)和1- BCY 2 -8-PBL-C 10 H ^ 6(5)(L = 2,制备了6-(Me 2 NCH 2)2 C 6 H 3)。化合物4和5允许研究Pb原子与Lewis酸性位点(BCy 2)或Lewis碱性位点(PPh 2)之间的相互作用。添加外部路易斯酸(BH 3 ·SMe的2)至5所提供的复杂的1-BCY 2 -8 - [(BH 3)2 L]的Pb-C 10 H ^ 6(7),在其中两个NME 2个基团的配位体L的由BH协调3。这个反应也被用于锡类似物1- BCY的合成2 -8 - [(BH 3)2 L]的Sn-C 10 H ^ 6(6)。新络合物4-7也与先前已知的,比较锡(II)类似物1-PPH 2 -8-SNL-C 10 H ^ 6(1),1-PPH 2 -8 - [(BH 3)2 L]的Sn-C 10 H ^ 6(2),1- BCY 2 -8-SNL-C 10 H ^ 6(3)。这组化合物使我们能够通过循环伏安法研究P→E和E→B原子接触对氧化还原电势E 1/2(ox1)的影响。其结果,E的选择性氧化(II)原子也进行了研究和新的有机锡(IV)硫化物[1-PPH 2 -8-LSn提(C 10 H ^ 6)(μ-S)] 2(9制备)时,锡(II)原子在1中的唯一氧化产物。除此之外,还计算了中性分子中P→E和E→B的周向接触,第一和第二电离能(IE)。因此,对所有可能的金属原子(Sn对Pb),路易斯酸(N→Sn / Pb对N(BH 3)Sn /)的组合,均采用密度泛函理论(DFT)进行了电子性能,完整的结构优化。Pb),周围取代基(BCy 2 vs. PPh 2),以及B3PW91 / 6-311 + g(2df,p)理论水平的三个分子电荷(0 vs. 1 + vs. 2 +)。
更新日期:2020-10-11
中文翻译:

取代萘中N-配位的Sn / Pb(II)原子上供体-受体键的研究:Pb→B相互作用的证据
围取代萘衍生物,即,Ñ配位的plumbylenes 1-PPH 2 -8-PBL-C 10 H ^ 6(4)和1- BCY 2 -8-PBL-C 10 H ^ 6(5)(L = 2,制备了6-(Me 2 NCH 2)2 C 6 H 3)。化合物4和5允许研究Pb原子与Lewis酸性位点(BCy 2)或Lewis碱性位点(PPh 2)之间的相互作用。添加外部路易斯酸(BH 3 ·SMe的2)至5所提供的复杂的1-BCY 2 -8 - [(BH 3)2 L]的Pb-C 10 H ^ 6(7),在其中两个NME 2个基团的配位体L的由BH协调3。这个反应也被用于锡类似物1- BCY的合成2 -8 - [(BH 3)2 L]的Sn-C 10 H ^ 6(6)。新络合物4-7也与先前已知的,比较锡(II)类似物1-PPH 2 -8-SNL-C 10 H ^ 6(1),1-PPH 2 -8 - [(BH 3)2 L]的Sn-C 10 H ^ 6(2),1- BCY 2 -8-SNL-C 10 H ^ 6(3)。这组化合物使我们能够通过循环伏安法研究P→E和E→B原子接触对氧化还原电势E 1/2(ox1)的影响。其结果,E的选择性氧化(II)原子也进行了研究和新的有机锡(IV)硫化物[1-PPH 2 -8-LSn提(C 10 H ^ 6)(μ-S)] 2(9制备)时,锡(II)原子在1中的唯一氧化产物。除此之外,还计算了中性分子中P→E和E→B的周向接触,第一和第二电离能(IE)。因此,对所有可能的金属原子(Sn对Pb),路易斯酸(N→Sn / Pb对N(BH 3)Sn /)的组合,均采用密度泛函理论(DFT)进行了电子性能,完整的结构优化。Pb),周围取代基(BCy 2 vs. PPh 2),以及B3PW91 / 6-311 + g(2df,p)理论水平的三个分子电荷(0 vs. 1 + vs. 2 +)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号