当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Electrochemical Reduction Products and Processes in Silica Anodes for Next‐Generation Lithium‐Ion Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-09-03 , DOI: 10.1002/aenm.202001826 Jake E. Entwistle 1 , Samuel G. Booth 1 , Dean S. Keeble 2 , Faisal Ayub 1 , Maximilian Yan 1 , Serena A. Corr 1, 3 , Denis J. Cumming 1 , Siddharth V. Patwardhan 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-09-03 , DOI: 10.1002/aenm.202001826 Jake E. Entwistle 1 , Samuel G. Booth 1 , Dean S. Keeble 2 , Faisal Ayub 1 , Maximilian Yan 1 , Serena A. Corr 1, 3 , Denis J. Cumming 1 , Siddharth V. Patwardhan 1
Affiliation
|
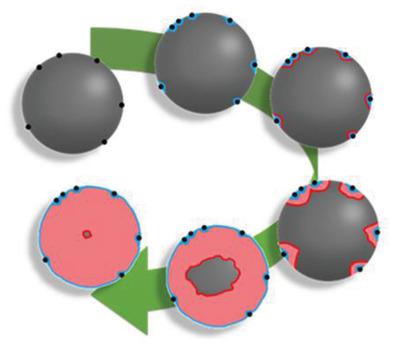
The use of silica as a lithium‐ion battery anode material requires a pretreatment step to induce electrochemical activity. The partially reversible electrochemical reduction reaction between silica and lithium has been postulated to produce silicon, which can subsequently reversibly react with lithium, providing stable capacities higher than graphite materials. Up to now, the electrochemical reduction pathway and the nature of the products were unknown, thereby hampering the design, optimization, and wider uptake of silica‐based anodes. Here, the electrochemical reduction pathway is uncovered and, for the first time, elemental silicon is identified as a reduction product. These insights, gleaned from analysis of the current response and capacity increase during reduction, conclusively demonstrated that silica must be reduced to introduce reversible capacity and the highest capacities of 600 mAh g−1 are achieved by using a constant load discharge at elevated temperature. Characterization via total scattering X‐ray pair distribution function analysis reveal the reduction products are amorphous in nature, highlighting the need for local structural methods to uncover vital information often inaccessible by traditional diffraction. These insights contribute toward understanding the electrochemical reduction of silica and can inform the development of pretreatment processes to enable their incorporation into next‐generation lithium‐ion batteries.
中文翻译:

下一代锂离子电池硅阳极中电化学还原产物和工艺的见解
使用二氧化硅作为锂离子电池负极材料需要进行预处理以诱导电化学活性。假定二氧化硅和锂之间的部分可逆的电化学还原反应可产生硅,随后可与锂可逆地反应,从而提供了比石墨材料更高的稳定容量。到目前为止,尚不知道电化学还原途径和产物的性质,从而阻碍了基于二氧化硅的阳极的设计,优化和更广泛的吸收。在此,未发现电化学还原途径,并且首次将元素硅鉴定为还原产物。这些见解来自对当前反应和减少过程中产能增加的分析,-1是通过在高温下使用恒定负载放电实现的。通过总散射X射线对分布函数分析的特征表明还原产物本质上是无定形的,这突出表明需要采用局部结构方法来发现传统衍射通常无法获得的重要信息。这些见解有助于理解二氧化硅的电化学还原,并可以为预处理工艺的发展提供信息,从而使它们能够并入下一代锂离子电池中。
更新日期:2020-09-03
中文翻译:

下一代锂离子电池硅阳极中电化学还原产物和工艺的见解
使用二氧化硅作为锂离子电池负极材料需要进行预处理以诱导电化学活性。假定二氧化硅和锂之间的部分可逆的电化学还原反应可产生硅,随后可与锂可逆地反应,从而提供了比石墨材料更高的稳定容量。到目前为止,尚不知道电化学还原途径和产物的性质,从而阻碍了基于二氧化硅的阳极的设计,优化和更广泛的吸收。在此,未发现电化学还原途径,并且首次将元素硅鉴定为还原产物。这些见解来自对当前反应和减少过程中产能增加的分析,-1是通过在高温下使用恒定负载放电实现的。通过总散射X射线对分布函数分析的特征表明还原产物本质上是无定形的,这突出表明需要采用局部结构方法来发现传统衍射通常无法获得的重要信息。这些见解有助于理解二氧化硅的电化学还原,并可以为预处理工艺的发展提供信息,从而使它们能够并入下一代锂离子电池中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号