Chemical Physics ( IF 2.0 ) Pub Date : 2020-09-04 , DOI: 10.1016/j.chemphys.2020.110978 Tao Xia , Dan Li , Longjiu Cheng
|
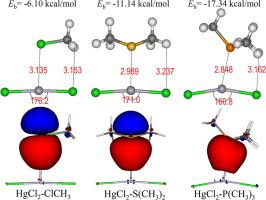
Halogen bonds have received increasing interest in non-covalent interactions. Recently, a new kind of non-covalent interaction, spodium bond, was proposed to refer to a net attractive interaction between any element of group 12 and electron rich atoms. Due to strong relativistic effect, Hg is much different from the other group 12 elements, which prefers more linear coordination. Thus, we theoretically studied the model of HgCl2⋯L (where L = ClR, SR2, PR3 families) to explore the nature of the linear coordinated spodium bonds. Analyses of electrostatic potential surfaces, together with ELF, LOL, and chemical bonding analyses, suggest the presence of covalent interaction. Complexes with nitrogen, oxygen, and fluorine donors result in more coulombic component, whereas others are dominated by covalent interaction, indicating coexistence of coulombic and covalent interaction. Besides, covalent interaction is significantly stronger with phosphorus donor. This model can provide intriguing perspectives for future weak intermolecular interactions studies.
中文翻译:

HgCl 2 ⋯L(L = ClR,SR 2和PR 3)二聚体中的钾键的理论分析
卤素键越来越受到非共价相互作用的关注。最近,提出了一种新的非共价相互作用,即bond键,是指第12组任何元素与富电子原子之间的净吸引相互作用。由于强烈的相对论效应,汞与其他12种元素有很大不同,后者更倾向于线性配合。因此,我们从理论上研究了HgCl 2 ⋯L的模型(其中L = ClR,SR 2,PR 3家族)以探索线性配位键的性质。静电势表面的分析以及ELF,LOL和化学键合分析表明存在共价相互作用。与氮,氧和氟供体的配合物导致更多的库仑组分,而其他化合物则以共价相互作用为主,表明库仑和共价相互作用共存。此外,与磷供体的共价相互作用明显更强。该模型可以为未来的弱分子间相互作用研究提供有趣的观点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号