Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge effects at nano-bio interfaces: a model of charged gold nanoclusters on amylin fibrillation.
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-03 , DOI: 10.1039/d0nr03877f Xintong Tang 1 , Guanbin Gao , Ting Zhang , Jianhang Li , Meng Yu , Meng He , Taolei Sun
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-03 , DOI: 10.1039/d0nr03877f Xintong Tang 1 , Guanbin Gao , Ting Zhang , Jianhang Li , Meng Yu , Meng He , Taolei Sun
Affiliation
|
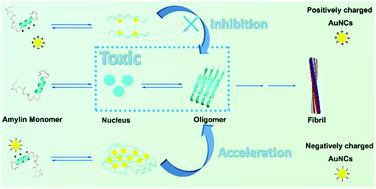
The misfolding and abnormal amyloid fibrillation of proteins/peptides are associated with more than 20 human diseases. Although dozens of nanoparticles have been investigated for the inhibition effect on the misfolding and fibrillation of pathogenesis-related proteins/peptides, there are few reports on charge effects of nano inhibitors on amyloid fibrillation. Herein, same-sized gold nanoclusters modified with 2-aminoethanethiol hydrochloride (CSH-AuNCs, positively charged in pH 7.4) or 3-mercaptopropionic acid (MPA-AuNCs, negatively charged in pH 7.4) were synthesized and adopted as models to explore the charge effect of nano inhibitors on amylin fibrillation at the nano-bio interface. ThT fluorescence kinetics analysis, AFM images and circular dichroism (CD) spectra showed that electropositive CSH-AuNCs inhibited the misfolding and fibrillation of amylin in a dosage-dependent manner, but electronegative MPA-AuNCs accelerated the misfolding and fibrillation of amylin in a dosage-dependent manner. Moreover, the theoretical and experimental results revealed the interaction mechanism between amylin and ligands of AuNCs at the nano-bio interfaces. Electropositive CSH-AuNCs could be bound to the main nucleating region of amylin via hydrogen bonding and endowed the nanocomplex with more positive net charges (amylin monomer with a positive +26.23 ± 0.80 mV zeta potential), which would inhibit the misfolding and aggregation of amylin via electrostatic repulsion and steric hindrance. In contrast, electronegative MPA-AuNCs could absorb electropositive amylin via strong electrostatic attractions, which accelerated the fibrillation process of amylin via enhancing local concentrations. Moreover, cell experiments showed that both the charged AuNCs had good biocompatibility and electronegetive MPA-AuNCs showed a better protective effect in the amylin-induced cell model than electropositive CSH-AuNCs. These results provide an insight into structure-based nanodrug design for protein conformational diseases.
中文翻译:

纳米生物界面上的电荷效应:带电金纳米簇对胰岛淀粉样蛋白原纤化的模型。
蛋白质/肽的错误折叠和淀粉样蛋白原纤维异常与20多种人类疾病有关。尽管已经研究了数十种纳米颗粒对致病相关蛋白/肽的错误折叠和原纤化的抑制作用,但是关于纳米抑制剂对淀粉样蛋白原纤化的电荷影响的报道很少。在此,合成了用2-氨基乙硫醇盐酸盐(CSH-AuNCs,在pH 7.4中带正电荷)或3-巯基丙酸(MPA-AuNCs,在pH 7.4中带负电荷)修饰的相同大小的金纳米簇。抑制剂对纳米生物界面上胰岛淀粉样蛋白原纤化的影响。ThT荧光动力学分析,AFM图像和圆二色性(CD)谱表明,正电CSH-AuNCs以剂量依赖的方式抑制胰岛淀粉样多肽的错误折叠和原纤维形成,而负电MPA-AuNCs以剂量依赖的方式加速淀粉样多肽的错误折叠和原纤维形成。此外,理论和实验结果揭示了胰岛淀粉样多肽与AuNCs配体在纳米生物界面上的相互作用机理。电阳性的CSH-AuNCs可以结合到胰岛淀粉样多肽的主要成核区域通过氢键结合并赋予纳米复合物更多的正净电荷(带有+26.23±0.80 mV zeta电位的正淀粉淀粉单体),这将通过静电排斥和空间位阻抑制胰淀素的错误折叠和聚集。与此相反,带负电的MPA-AuNCs可以吸收正电糊精通过强静电引力,这加速了胰岛淀粉样多肽的原纤化过程通过提高局部浓度。此外,细胞实验表明,两个带电的AuNCs都具有良好的生物相容性,而电性MPA-AuNCs在胰岛淀粉样多肽诱导的细胞模型中显示出比电正性CSH-AuNCs更好的保护作用。这些结果为蛋白质构象疾病的基于结构的纳米药物设计提供了见识。
更新日期:2020-09-24
中文翻译:

纳米生物界面上的电荷效应:带电金纳米簇对胰岛淀粉样蛋白原纤化的模型。
蛋白质/肽的错误折叠和淀粉样蛋白原纤维异常与20多种人类疾病有关。尽管已经研究了数十种纳米颗粒对致病相关蛋白/肽的错误折叠和原纤化的抑制作用,但是关于纳米抑制剂对淀粉样蛋白原纤化的电荷影响的报道很少。在此,合成了用2-氨基乙硫醇盐酸盐(CSH-AuNCs,在pH 7.4中带正电荷)或3-巯基丙酸(MPA-AuNCs,在pH 7.4中带负电荷)修饰的相同大小的金纳米簇。抑制剂对纳米生物界面上胰岛淀粉样蛋白原纤化的影响。ThT荧光动力学分析,AFM图像和圆二色性(CD)谱表明,正电CSH-AuNCs以剂量依赖的方式抑制胰岛淀粉样多肽的错误折叠和原纤维形成,而负电MPA-AuNCs以剂量依赖的方式加速淀粉样多肽的错误折叠和原纤维形成。此外,理论和实验结果揭示了胰岛淀粉样多肽与AuNCs配体在纳米生物界面上的相互作用机理。电阳性的CSH-AuNCs可以结合到胰岛淀粉样多肽的主要成核区域通过氢键结合并赋予纳米复合物更多的正净电荷(带有+26.23±0.80 mV zeta电位的正淀粉淀粉单体),这将通过静电排斥和空间位阻抑制胰淀素的错误折叠和聚集。与此相反,带负电的MPA-AuNCs可以吸收正电糊精通过强静电引力,这加速了胰岛淀粉样多肽的原纤化过程通过提高局部浓度。此外,细胞实验表明,两个带电的AuNCs都具有良好的生物相容性,而电性MPA-AuNCs在胰岛淀粉样多肽诱导的细胞模型中显示出比电正性CSH-AuNCs更好的保护作用。这些结果为蛋白质构象疾病的基于结构的纳米药物设计提供了见识。









































 京公网安备 11010802027423号
京公网安备 11010802027423号