当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent-driven isomerization of cis,cis-muconic acid for the production of specialty and performance-advantaged cyclic biobased monomers
Green Chemistry ( IF 9.3 ) Pub Date : 2020-09-03 , DOI: 10.1039/d0gc02108c Jack M. Carraher 1, 2, 3, 4, 5 , Prerana Carter 1, 2, 3, 4, 5 , Radhika G. Rao 1, 2, 3, 4, 5 , Michael J. Forrester 1, 2, 3, 4 , Toni Pfennig 1, 2, 3, 4, 5 , Brent H. Shanks 1, 2, 3, 4, 5 , Eric W. Cochran 1, 2, 3, 4 , Jean-Philippe Tessonnier 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020-09-03 , DOI: 10.1039/d0gc02108c Jack M. Carraher 1, 2, 3, 4, 5 , Prerana Carter 1, 2, 3, 4, 5 , Radhika G. Rao 1, 2, 3, 4, 5 , Michael J. Forrester 1, 2, 3, 4 , Toni Pfennig 1, 2, 3, 4, 5 , Brent H. Shanks 1, 2, 3, 4, 5 , Eric W. Cochran 1, 2, 3, 4 , Jean-Philippe Tessonnier 1, 2, 3, 4, 5
Affiliation
|
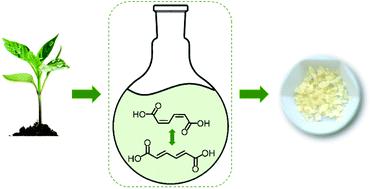
The quest for green plastics calls for new routes to aromatic monomers using biomass as a feedstock. Suitable feedstock molecules and conversion pathways have already been identified for several commodity aromatics through retrosynthetic analysis. However, this approach suffers from some limitations as it targets a single molecule at a time. A more impactful approach would be to target bioprivileged molecules that are intermediates to an array of commodity and specialty chemicals along with novel compounds. Muconic acid (MA) has recently been identified as a bioprivileged intermediate as it gives access to valuable aliphatic and cyclic diacid monomers including terephthalic acid (TPA), 1,4-cyclohexanedicarboxylic acid (CHDA), and novel monounsaturated 1,4-cyclohexenedicarboxylic acids (CH1DA, CH2DA). However, accessing these cyclic monomers from MA requires to first isomerize biologically-produced cis,cis-MA to Diels–Alder active trans,trans-MA. A major impediment in this isomerization is the irreversible ring closing of MA to produce lactones. Herein, we demonstrate a green solvent-mediated isomerization using dimethyl sulfoxide and water. The mechanistic understanding achieved here elucidates the role of low concentrations of water in reducing the acidity of the system, thereby preventing the formation of lactones and improving the selectivity to trans,trans-MA from less than 5% to over 85%. Finally, a Diels–Alder reaction with trans,trans-MA is demonstrated with ethylene. The monounsaturated cyclic diacid obtained through this reaction (CH1DA) can be converted in a single step into TPA and CHDA, or can be directly copolymerized with adipic acid and hexamethylenediamine to tailor the thermal and mechanical properties of conventional Nylon 6,6.
中文翻译:

溶剂驱动的顺式,顺式-粘康酸异构化,用于生产特种和性能优越的环状生物基单体
对绿色塑料的追求要求使用生物质作为原料的新途径来生产芳族单体。通过逆合成分析已经确定了几种商品芳烃的合适原料分子和转化途径。然而,这种方法由于一次靶向单个分子而受到一些限制。更具影响力的方法是针对生物特权分子,这些分子是一系列商品和特种化学品以及新型化合物的中间产物。粘康酸(MA)最近被鉴定为生物特权中间体,因为它可以获取有价值的脂族和环状二酸单体,包括对苯二甲酸(TPA),1,4-环己烷二羧酸(CHDA)和新型单不饱和1,4-环己二羧酸(CH1DA,CH2DA)。然而,顺式,顺式-MA转化为Diels-Alder活性反式,反式-MA。这种异构化的主要障碍是MA产生内酯的不可逆闭环。在本文中,我们证明了使用二甲基亚砜和水的绿色溶剂介导的异构化。在此获得的机理理解阐明了低浓度水在降低系统酸度中的作用,从而防止了内酯的形成并将反式,反式-MA的选择性从不到5%提高到超过85%。最后,与反式,反式的狄尔斯-阿尔德反应-MA用乙烯证明。通过此反应获得的单不饱和环状二酸(CH1DA)可以一步转化为TPA和CHDA,也可以直接与己二酸和六亚甲基二胺共聚,以调节常规尼龙6,6的热和机械性能。
更新日期:2020-10-05
中文翻译:

溶剂驱动的顺式,顺式-粘康酸异构化,用于生产特种和性能优越的环状生物基单体
对绿色塑料的追求要求使用生物质作为原料的新途径来生产芳族单体。通过逆合成分析已经确定了几种商品芳烃的合适原料分子和转化途径。然而,这种方法由于一次靶向单个分子而受到一些限制。更具影响力的方法是针对生物特权分子,这些分子是一系列商品和特种化学品以及新型化合物的中间产物。粘康酸(MA)最近被鉴定为生物特权中间体,因为它可以获取有价值的脂族和环状二酸单体,包括对苯二甲酸(TPA),1,4-环己烷二羧酸(CHDA)和新型单不饱和1,4-环己二羧酸(CH1DA,CH2DA)。然而,顺式,顺式-MA转化为Diels-Alder活性反式,反式-MA。这种异构化的主要障碍是MA产生内酯的不可逆闭环。在本文中,我们证明了使用二甲基亚砜和水的绿色溶剂介导的异构化。在此获得的机理理解阐明了低浓度水在降低系统酸度中的作用,从而防止了内酯的形成并将反式,反式-MA的选择性从不到5%提高到超过85%。最后,与反式,反式的狄尔斯-阿尔德反应-MA用乙烯证明。通过此反应获得的单不饱和环状二酸(CH1DA)可以一步转化为TPA和CHDA,也可以直接与己二酸和六亚甲基二胺共聚,以调节常规尼龙6,6的热和机械性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号