当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Ni3S2 catalysts using various sulphur sources and their HER and OER performances
CrystEngComm ( IF 2.6 ) Pub Date : 2020-09-03 , DOI: 10.1039/d0ce01015d Xiaowei Lv 1, 2, 3, 4, 5 , Palanisamy Kannan 5, 6, 7, 8, 9 , Shan Ji 5, 6, 7, 8, 9 , Xuyun Wang 1, 2, 3, 4, 5 , Hui Wang 1, 2, 3, 4, 5
CrystEngComm ( IF 2.6 ) Pub Date : 2020-09-03 , DOI: 10.1039/d0ce01015d Xiaowei Lv 1, 2, 3, 4, 5 , Palanisamy Kannan 5, 6, 7, 8, 9 , Shan Ji 5, 6, 7, 8, 9 , Xuyun Wang 1, 2, 3, 4, 5 , Hui Wang 1, 2, 3, 4, 5
Affiliation
|
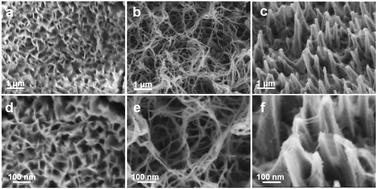
It is highly anticipated that hydrogen can be produced via water splitting, and can be electrocatalyzed using non-precious electrocatalysts. Accordingly, nickel sulfide (Ni3S2) has attracted considerable attention due to its high electrochemical performance for the hydrogen evolution reaction and oxygen evolution reaction. In this study, nano-sized Ni3S2 binder-free electrodes were directly grown on Ni foam using various methods. The obtained results showed that the interconnected sheet-like Ni3S2 with a hydrophilic surface, which was prepared from Na2S via the hydrothermal method, exhibited the best HER and OER electrocatalytic performance. Its thin nanosheet structure could offer more active centers for the HER and OER in the aqueous alkaline electrolyte and its hydrophilic surface could also facilitate the transfer of ions at the interface of the electrode and electrolyte. The best Ni3S2 electrode synthesized using Na2S exhibited an overpotential of 43 mV and 127 mV at the current density of 10 mA cm−2 and 100 mA cm−2, respectively. Furthermore, it also exhibited superior electrochemical activity and durability for the OER.
中文翻译:

利用多种硫源合成Ni3S2催化剂及其HER和OER性能
高度期望的是氢可以通过水分解产生,并且可以使用非贵重的电催化剂进行电催化。因此,硫化镍(Ni 3 S 2)由于其对于氢释放反应和氧释放反应的高电化学性能而引起了相当大的关注。在这项研究中,使用各种方法将纳米级Ni 3 S 2无粘结剂电极直接在泡沫镍上生长。所得结果表明,由Na 2 S经以下步骤制备的具有亲水性表面的互连片状Ni 3 S 2:水热法表现出最好的HER和OER电催化性能。它的薄纳米片结构可以为碱性电解质水溶液中的HER和OER提供更多的活性中心,并且其亲水性表面还可以促进离子在电极和电解质界面处的转移。使用Na 2 S合成的最佳Ni 3 S 2电极在电流密度分别为10 mA cm -2和100 mA cm -2时分别表现出43 mV和127 mV的过电势。此外,它还显示出优异的电化学活性和对OER的耐久性。
更新日期:2020-10-12
中文翻译:

利用多种硫源合成Ni3S2催化剂及其HER和OER性能
高度期望的是氢可以通过水分解产生,并且可以使用非贵重的电催化剂进行电催化。因此,硫化镍(Ni 3 S 2)由于其对于氢释放反应和氧释放反应的高电化学性能而引起了相当大的关注。在这项研究中,使用各种方法将纳米级Ni 3 S 2无粘结剂电极直接在泡沫镍上生长。所得结果表明,由Na 2 S经以下步骤制备的具有亲水性表面的互连片状Ni 3 S 2:水热法表现出最好的HER和OER电催化性能。它的薄纳米片结构可以为碱性电解质水溶液中的HER和OER提供更多的活性中心,并且其亲水性表面还可以促进离子在电极和电解质界面处的转移。使用Na 2 S合成的最佳Ni 3 S 2电极在电流密度分别为10 mA cm -2和100 mA cm -2时分别表现出43 mV和127 mV的过电势。此外,它还显示出优异的电化学活性和对OER的耐久性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号