当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Helix-Coil Transition at a Glycine Following a Nascent α-Helix: A Synergetic Guidance Mechanism for Helix Growth.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.jpca.0c05489 Sunit Pal 1 , Shreya Banerjee 1 , Erode N Prabhakaran 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.jpca.0c05489 Sunit Pal 1 , Shreya Banerjee 1 , Erode N Prabhakaran 1
Affiliation
|
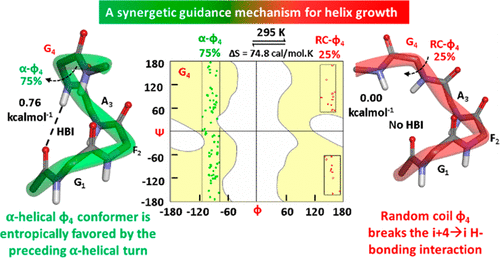
A detailed understanding of forces guiding the rapid folding of a polypeptide from an apparently random coil state to an ordered α-helical structure following the rate-limiting preorganization of the initial three residue backbones into helical conformation is imperative to comprehending and regulating protein folding and for the rational design of biological mimetics. However, several details of this process are still unknown. First, although the helix–coil transition was proposed to originate at the residue level (J. Chem. Phys.1959,31, 526–535; J. Chem. Phys.1961,34, 1963–1974), all helix-folding studies have only established it between time-averaged bulk states of a long-lived helix and several transiently populated random coils, along the whole helix model sequence. Second, the predominant thermodynamic forces driving either this two-state transition or the faster helix growth following helix nucleation are still unclear. Third, the conformational space of the random coil state is not well-defined unlike its corresponding α-helix. Here we investigate the restrictions placed on the conformational space of a Gly residue backbone, as a result of it immediately succeeding a nascent α-helical turn. Analyses of the temperature-dependent 1D-, 2D-NMR, FT-IR, and CD spectra and GROMACS MD simulation trajectory of a Gly residue backbone following a model α-helical turn, which is artificially rigidified by a covalent hydrogen bond surrogate, reveal that: (i) the α-helical turn guides the ϕ torsion of the Gly exclusively into either a predominantly populated entropically favored α-helical (α-ϕ) state or a scarcely populated random coil (RC-ϕ) state; (ii) the α-ϕ state of Gly in turn favors the stability of the preceding α-helical turn, while the RC-ϕ state disrupts it, revealing an entropy-driven synergetic guidance for helix growth in the residue following helix nucleation. The applicability of a current synergetic guidance mechanism to explain rapid helix growth in folded and unfolded states of proteins and helical peptides is discussed.
中文翻译:

新生的α-螺旋后甘氨酸的螺旋-螺旋过渡:螺旋生长的协同引导机制。
在最初的三个残基主链进行限速预组织后,对引导多肽从明显随机的卷曲状态快速折叠到有序的α-螺旋结构的力的详细理解对于理解和调节蛋白质折叠是必不可少的。拟态模拟物的合理设计。但是,此过程的一些细节仍然未知。首先,尽管螺旋线圈过渡是拟在该残基电平(以发起J.化学物理。1959,31,526-535; J。化学物理。1961年,34,1963–1974年),所有螺旋折叠研究仅将其建立在整个螺旋模型序列的长寿命螺旋的时间平均体态与几个瞬时填充的随机线圈之间。其次,尚不清楚驱动此状态过渡或螺旋成核后螺旋生长更快的主要热力学力。第三,随机线圈状态的构象空间与其对应的α-螺旋不同,没有得到很好的定义。在这里,我们研究了对Gly残基主链构象空间的限制,因为它紧随新生α-螺旋转弯而形成。根据模型α螺旋转弯分析Gly残基骨架的随温度变化的1D-,2D-NMR,FT-IR和CD光谱以及GROMACS MD模拟轨迹,通过共价氢键替代物人工硬化的结果表明:(i)α螺旋转向将Gly的exclusively扭曲专门引导到主要是熵集中的α螺旋(α-ϕ)状态或很少有人居住的状态随机线圈(RC-ϕ)状态;(ii)Gly的α-ϕ状态反过来又有利于先前的α-螺旋匝的稳定性,而RC-s状态则破坏了它的稳定性,从而揭示了由熵驱动的协同指导,指导螺旋成核后残基中的螺旋生长。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。(i)α螺旋圈将Gly的exclusively扭转专门引导到主要是熵集中的α螺旋(α-ϕ)状态或很少有人居住的随机线圈(RC-ϕ)状态;(ii)Gly的α-ϕ状态反过来又有利于先前的α-螺旋匝的稳定性,而RC-s状态则破坏了它的稳定性,从而揭示了由熵驱动的协同指导,指导螺旋成核后残基中的螺旋生长。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。(i)α螺旋圈将Gly的exclusively扭转专门引导到主要是熵集中的α螺旋(α-ϕ)状态或很少有人居住的随机线圈(RC-ϕ)状态;(ii)Gly的α-ϕ状态反过来又有利于先前的α-螺旋匝的稳定性,而RC-s状态则破坏了它的稳定性,从而揭示了由熵驱动的协同指导,指导螺旋成核后残基中的螺旋生长。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。揭示了螺旋成核后残基中螺旋生长的熵驱动协同指导。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。揭示了螺旋成核后残基中螺旋生长的熵驱动协同指导。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。
更新日期:2020-09-18
中文翻译:

新生的α-螺旋后甘氨酸的螺旋-螺旋过渡:螺旋生长的协同引导机制。
在最初的三个残基主链进行限速预组织后,对引导多肽从明显随机的卷曲状态快速折叠到有序的α-螺旋结构的力的详细理解对于理解和调节蛋白质折叠是必不可少的。拟态模拟物的合理设计。但是,此过程的一些细节仍然未知。首先,尽管螺旋线圈过渡是拟在该残基电平(以发起J.化学物理。1959,31,526-535; J。化学物理。1961年,34,1963–1974年),所有螺旋折叠研究仅将其建立在整个螺旋模型序列的长寿命螺旋的时间平均体态与几个瞬时填充的随机线圈之间。其次,尚不清楚驱动此状态过渡或螺旋成核后螺旋生长更快的主要热力学力。第三,随机线圈状态的构象空间与其对应的α-螺旋不同,没有得到很好的定义。在这里,我们研究了对Gly残基主链构象空间的限制,因为它紧随新生α-螺旋转弯而形成。根据模型α螺旋转弯分析Gly残基骨架的随温度变化的1D-,2D-NMR,FT-IR和CD光谱以及GROMACS MD模拟轨迹,通过共价氢键替代物人工硬化的结果表明:(i)α螺旋转向将Gly的exclusively扭曲专门引导到主要是熵集中的α螺旋(α-ϕ)状态或很少有人居住的状态随机线圈(RC-ϕ)状态;(ii)Gly的α-ϕ状态反过来又有利于先前的α-螺旋匝的稳定性,而RC-s状态则破坏了它的稳定性,从而揭示了由熵驱动的协同指导,指导螺旋成核后残基中的螺旋生长。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。(i)α螺旋圈将Gly的exclusively扭转专门引导到主要是熵集中的α螺旋(α-ϕ)状态或很少有人居住的随机线圈(RC-ϕ)状态;(ii)Gly的α-ϕ状态反过来又有利于先前的α-螺旋匝的稳定性,而RC-s状态则破坏了它的稳定性,从而揭示了由熵驱动的协同指导,指导螺旋成核后残基中的螺旋生长。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。(i)α螺旋圈将Gly的exclusively扭转专门引导到主要是熵集中的α螺旋(α-ϕ)状态或很少有人居住的随机线圈(RC-ϕ)状态;(ii)Gly的α-ϕ状态反过来又有利于先前的α-螺旋匝的稳定性,而RC-s状态则破坏了它的稳定性,从而揭示了由熵驱动的协同指导,指导螺旋成核后残基中的螺旋生长。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。揭示了螺旋成核后残基中螺旋生长的熵驱动协同指导。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。揭示了螺旋成核后残基中螺旋生长的熵驱动协同指导。讨论了目前的协同指导机制,以解释在蛋白质和螺旋肽的折叠和未折叠状态下快速螺旋生长的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号