当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Splitting Methyl Ketones into Two Parts: Synthesis of 4(3H)-Quinazolinones via Consecutive Cyclization/Ring-Opening Reaction.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02415 Peng Zhao 1 , Xiao-Xiao Yu 1 , You Zhou 1 , Xiao Geng 1 , Can Wang 1 , Chun Huang 1 , Yan-Dong Wu 1 , Yan-Ping Zhu 2 , An-Xin Wu 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02415 Peng Zhao 1 , Xiao-Xiao Yu 1 , You Zhou 1 , Xiao Geng 1 , Can Wang 1 , Chun Huang 1 , Yan-Dong Wu 1 , Yan-Ping Zhu 2 , An-Xin Wu 1
Affiliation
|
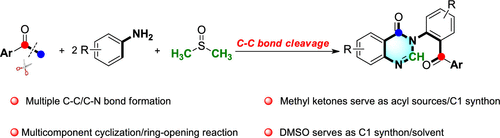
The first synthesis of arylquinazolinones has been disclosed via the consecutive oxidative cyclization/ring-opening of anilines and methyl ketones. This transformation is characterized by the splitting of the methyl ketone as acyl and C1 sources through C(CO)–C(sp3) bond cleavage, using DMSO as a C1 source via C–S bond cleavage, which expands the use of methyl ketones as valuable building blocks and enriches the library of preparation quinazolinones. Moreover, preliminary mechanistic studies indicate that this multicomponent reaction underwent a consecutive cyclization/ring-opening sequence.
中文翻译:

将甲基酮分为两部分:通过连续环化/开环反应合成4(3H)-喹唑啉酮。
已经通过苯胺和甲基酮的连续氧化环化/开环公开了芳基喹唑啉酮的第一合成。这种转变的特征是通过C(CO)–C(sp 3)键裂解将甲基酮分解为酰基和C1来源,使用DMSO通过C–S键裂解将DMSO作为C1来源,这扩大了甲基酮的使用作为有价值的组成部分,并丰富了喹唑啉酮制备库。此外,初步的机理研究表明,该多组分反应经历了连续的环化/开环序列。
更新日期:2020-09-20
中文翻译:

将甲基酮分为两部分:通过连续环化/开环反应合成4(3H)-喹唑啉酮。
已经通过苯胺和甲基酮的连续氧化环化/开环公开了芳基喹唑啉酮的第一合成。这种转变的特征是通过C(CO)–C(sp 3)键裂解将甲基酮分解为酰基和C1来源,使用DMSO通过C–S键裂解将DMSO作为C1来源,这扩大了甲基酮的使用作为有价值的组成部分,并丰富了喹唑啉酮制备库。此外,初步的机理研究表明,该多组分反应经历了连续的环化/开环序列。











































 京公网安备 11010802027423号
京公网安备 11010802027423号