当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
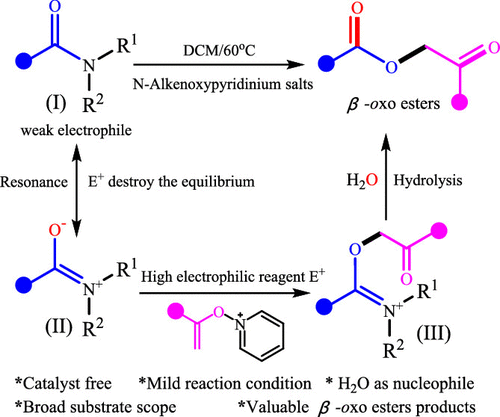
A Strategy for Amide to β-Oxo Ester Transformation via N-Alkenoxypyridinium Salts as the Activator and H2O as the Nucleophile.
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02457 Nan Wu 1 , Chuang Li 1 , Jiajia Mi 1 , Yan Zheng 1 , Zhou Xu 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02457 Nan Wu 1 , Chuang Li 1 , Jiajia Mi 1 , Yan Zheng 1 , Zhou Xu 1
Affiliation
|
N-Alkenoxypyridinium salts were found to be highly active electrophilic reagents that could be used to activate the C–N bond of amides. Both aromatic amides and aliphatic amides could be transformed into the corresponding β-oxo esters with good yields via the combined use of N-alkenoxypyridinium salts and water. The methodology proceeds under mild reaction conditions and is tolerant of various functional groups in both reaction partners.
中文翻译:

一种通过N-烷氧基吡啶鎓盐作为活化剂和H2O作为亲核试剂的酰胺转化为β-氧代酯的策略。
N-烯氧基吡啶鎓盐被发现是高度活性的亲电试剂,可用于激活酰胺的C–N键。通过结合使用N-烯氧基吡啶鎓盐和水,芳族酰胺和脂族酰胺都可以良好的产率转化为相应的β-氧代酯。该方法在温和的反应条件下进行,并且可以耐受两个反应伙伴中的各种官能团。
更新日期:2020-09-20
中文翻译:

一种通过N-烷氧基吡啶鎓盐作为活化剂和H2O作为亲核试剂的酰胺转化为β-氧代酯的策略。
N-烯氧基吡啶鎓盐被发现是高度活性的亲电试剂,可用于激活酰胺的C–N键。通过结合使用N-烯氧基吡啶鎓盐和水,芳族酰胺和脂族酰胺都可以良好的产率转化为相应的β-氧代酯。该方法在温和的反应条件下进行,并且可以耐受两个反应伙伴中的各种官能团。



























 京公网安备 11010802027423号
京公网安备 11010802027423号