当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-Neutral 1,3-Diol Synthesis by Base-Promoted Diastereoselective Alcohol-Aldolization.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02536 Na Shao 1 , Jean Rodriguez 1 , Adrien Quintard 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02536 Na Shao 1 , Jean Rodriguez 1 , Adrien Quintard 1
Affiliation
|
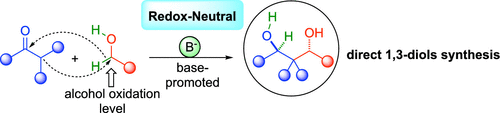
In order to prepare more efficiently key 1,3-diol fragments, we have devised a base-promoted redox-neutral condensation of ketones with alcohols. This diastereoselective alcohol–aldolization enables bypassing the classical oxidation and reduction steps necessary for the preparation of this crucial backbone by an overall redox-neutral formal borrowing hydrogen process. The starting alcohols constitute both the precursors of the in situ generated reactive aldehydes and the hydride source necessary for the chemoselective reduction of the aldol adduct intermediates.
中文翻译:

碱促进的非对映选择性醇醛缩合反应合成氧化还原中性1,3-二醇。
为了更有效地制备关键的1,3-二醇片段,我们设计了一种碱促进的酮与醇的氧化还原中性缩合反应。这种非对映选择性的醇-醛缩醛化反应可以绕过整个氧化还原中性的正式借用氢过程,从而绕开了制备该关键骨架所需的经典氧化和还原步骤。起始醇既构成原位生成的反应性醛的前体,又是化学选择性还原羟醛加合物中间产物所必需的氢化物源。
更新日期:2020-09-20
中文翻译:

碱促进的非对映选择性醇醛缩合反应合成氧化还原中性1,3-二醇。
为了更有效地制备关键的1,3-二醇片段,我们设计了一种碱促进的酮与醇的氧化还原中性缩合反应。这种非对映选择性的醇-醛缩醛化反应可以绕过整个氧化还原中性的正式借用氢过程,从而绕开了制备该关键骨架所需的经典氧化和还原步骤。起始醇既构成原位生成的反应性醛的前体,又是化学选择性还原羟醛加合物中间产物所必需的氢化物源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号