当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Electron Doping of Layered Metal Hydroxide Nanoplates (M = Co, Ni) Using n-Butyllithium.
Nano Letters ( IF 9.6 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.nanolett.0c03092 Eve Y Martinez 1 , Kuixin Zhu 1 , Christina W Li 1
Nano Letters ( IF 9.6 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.nanolett.0c03092 Eve Y Martinez 1 , Kuixin Zhu 1 , Christina W Li 1
Affiliation
|
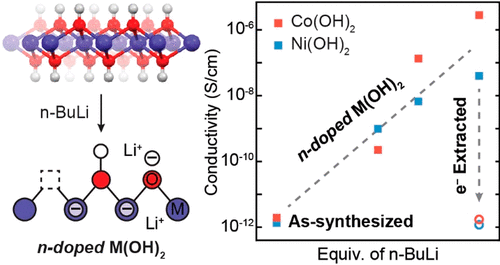
Ambipolar doping of metal oxides is critical toward broadening the functionality of semiconducting oxides in electronic devices. Most metal oxides, however, show a strong preference for a single doping polarity due to the intrinsic stability of particular defects in an oxide lattice. In this work, we demonstrate that layered metal hydroxide nanomaterials of Co and Ni, which are intrinsically p-doped in their anhydrous rock salt form, can be n-doped using n-BuLi as a strong electron donor. A combination of X-ray characterization techniques reveal that hydroxide vacancy formation, Li+ adsorption, and varying degrees of electron delocalization are responsible for the stability of injected electrons. The doped electrons induce conductivity increases of 4–6 orders of magnitude relative to the undoped M(OH)2. We anticipate that chemical electron doping of layered metal hydroxides may be a general strategy to increase carrier concentration and stability for n-doping of intrinsically p-type metal oxides.
中文翻译:

使用正丁基锂对层状金属氢氧化物纳米板(M = Co,Ni)进行可逆电子掺杂。
金属氧化物的双极性掺杂对于扩大电子设备中半导体氧化物的功能至关重要。然而,由于氧化物晶格中特定缺陷的固有稳定性,大多数金属氧化物对单一掺杂极性表现出强烈的偏好。在这项工作中,我们证明了钴和镍的层状金属氢氧化物纳米材料,它们以无水盐形式固有地p掺杂,可以使用n -BuLi作为强电子给体进行n掺杂。X射线表征技术的组合显示氢氧化物空位形成Li +吸附和不同程度的电子离域是注入电子的稳定性的原因。相对于未掺杂的M(OH)2,掺杂的电子引起的电导率增加4-6个数量级。我们预期对层状金属氢氧化物进行化学电子掺杂可能是提高载流子浓度和对本质上为p型金属氧化物进行n掺杂的稳定性的一般策略。
更新日期:2020-10-15
中文翻译:

使用正丁基锂对层状金属氢氧化物纳米板(M = Co,Ni)进行可逆电子掺杂。
金属氧化物的双极性掺杂对于扩大电子设备中半导体氧化物的功能至关重要。然而,由于氧化物晶格中特定缺陷的固有稳定性,大多数金属氧化物对单一掺杂极性表现出强烈的偏好。在这项工作中,我们证明了钴和镍的层状金属氢氧化物纳米材料,它们以无水盐形式固有地p掺杂,可以使用n -BuLi作为强电子给体进行n掺杂。X射线表征技术的组合显示氢氧化物空位形成Li +吸附和不同程度的电子离域是注入电子的稳定性的原因。相对于未掺杂的M(OH)2,掺杂的电子引起的电导率增加4-6个数量级。我们预期对层状金属氢氧化物进行化学电子掺杂可能是提高载流子浓度和对本质上为p型金属氧化物进行n掺杂的稳定性的一般策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号