当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral 1,3,2-Oxazaborolidine Catalysts for Enantioselective Photochemical Reactions.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-03 , DOI: 10.1021/acs.accounts.0c00379 Daniel P Schwinger 1 , Thorsten Bach 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-03 , DOI: 10.1021/acs.accounts.0c00379 Daniel P Schwinger 1 , Thorsten Bach 1
Affiliation
|
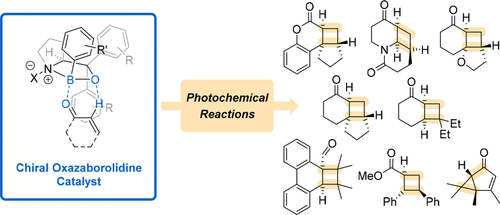
Asymmetric synthesis has posed a significant challenge to organic chemists for over a century. Several strategies have been developed to synthesize enantiomerically enriched compounds, which are ubiquitous in the pharmaceutical and agrochemical industries. While many organometallic and organic catalysts have been found to mediate thermal enantioselective reactions, the field of photochemistry lacks similar depth. Recently, chiral 1,3,2-oxazaborolidines have made the transition from Lewis acids that were exclusively applied to thermal reactions to catalysts for enantioselective photochemical reactions. Due to their modular structure, various 1,3,2-oxazaborolidines are readily available and can be easily fitted to a given chemical transformation. Their use holds great promise for future developments in photochemistry. This Account gives an overview of the substrate classes that are known to undergo enantioselective photochemical transformations in the presence of chiral 1,3,2-oxazaborolidines and touches on the catalytic mode of action, on the proposed enantiodifferentiation mechanism, as well as on recent computational studies.
中文翻译:

用于对映选择性光化学反应的手性1,3,2-氧杂硼硼烷催化剂。
一个多世纪以来,不对称合成对有机化学家提出了重大挑战。已经开发了几种策略来合成对映体富集的化合物,其在制药和农业化学工业中是普遍存在的。虽然已经发现许多有机金属和有机催化剂介导热对映选择性反应,但是光化学领域缺乏相似的深度。最近,手性的1,3,2-恶唑硼烷已从专门用于热反应的路易斯酸转变为对映选择性光化学反应的催化剂。由于它们的模块化结构,各种1,3,2-恶唑硼烷很容易获得,并且可以轻松地适应给定的化学转化。它们的使用对光化学的未来发展具有广阔的前景。
更新日期:2020-09-15
中文翻译:

用于对映选择性光化学反应的手性1,3,2-氧杂硼硼烷催化剂。
一个多世纪以来,不对称合成对有机化学家提出了重大挑战。已经开发了几种策略来合成对映体富集的化合物,其在制药和农业化学工业中是普遍存在的。虽然已经发现许多有机金属和有机催化剂介导热对映选择性反应,但是光化学领域缺乏相似的深度。最近,手性的1,3,2-恶唑硼烷已从专门用于热反应的路易斯酸转变为对映选择性光化学反应的催化剂。由于它们的模块化结构,各种1,3,2-恶唑硼烷很容易获得,并且可以轻松地适应给定的化学转化。它们的使用对光化学的未来发展具有广阔的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号