当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single‐molecule analysis reveals two distinct states of the compressed RecA filament on single‐stranded DNA
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-18 , DOI: 10.1002/1873-3468.13922 Aleksandr Alekseev 1 , Maksim Serdakov 1 , Georgii Pobegalov 1 , Alexandr Yakimov 1, 2 , Irina Bakhlanova 2 , Dmitry Baitin 2 , Mikhail Khodorkovskii 1, 3
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-18 , DOI: 10.1002/1873-3468.13922 Aleksandr Alekseev 1 , Maksim Serdakov 1 , Georgii Pobegalov 1 , Alexandr Yakimov 1, 2 , Irina Bakhlanova 2 , Dmitry Baitin 2 , Mikhail Khodorkovskii 1, 3
Affiliation
|
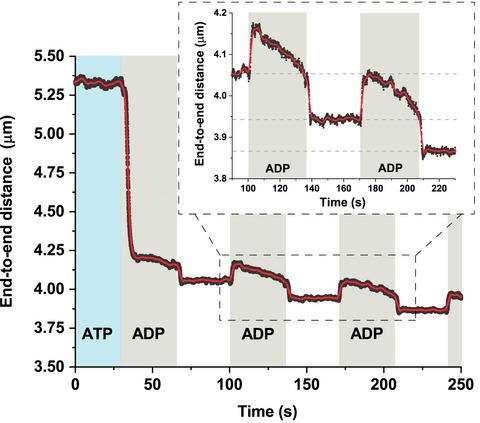
The RecA protein plays a key role in bacterial homologous recombination (HR) and acts through assembly of long helical filaments around single‐stranded DNA in the presence of ATP. Large‐scale conformational changes induced by ATP hydrolysis result in transitions between stretched and compressed forms of the filament. Here, using a single‐molecule approach, we show that compressed RecA nucleoprotein filaments can exist in two distinct interconvertible states depending on the presence of ADP in the monomer–monomer interface. Binding of ADP promotes cooperative conformational transitions and directly affects mechanical properties of the filament. Our findings reveal that RecA nucleoprotein filaments are able to continuously cycle between three mechanically distinct states that might have important implications for RecA‐mediated processes of HR.
中文翻译:

单分子分析揭示了单链 DNA 上压缩 RecA 细丝的两种不同状态
RecA 蛋白在细菌同源重组 (HR) 中起关键作用,并在 ATP 存在下通过在单链 DNA 周围组装长螺旋丝发挥作用。由 ATP 水解引起的大规模构象变化导致长丝的拉伸和压缩形式之间的转变。在这里,我们使用单分子方法表明,根据单体-单体界面中 ADP 的存在,压缩的 RecA 核蛋白丝可以以两种不同的相互转化状态存在。ADP 的结合促进了协同构象转变并直接影响长丝的机械性能。我们的研究结果表明,RecA 核蛋白丝能够在三种不同的机械状态之间连续循环,这可能对 RecA 介导的 HR 过程具有重要意义。
更新日期:2020-09-18
中文翻译:

单分子分析揭示了单链 DNA 上压缩 RecA 细丝的两种不同状态
RecA 蛋白在细菌同源重组 (HR) 中起关键作用,并在 ATP 存在下通过在单链 DNA 周围组装长螺旋丝发挥作用。由 ATP 水解引起的大规模构象变化导致长丝的拉伸和压缩形式之间的转变。在这里,我们使用单分子方法表明,根据单体-单体界面中 ADP 的存在,压缩的 RecA 核蛋白丝可以以两种不同的相互转化状态存在。ADP 的结合促进了协同构象转变并直接影响长丝的机械性能。我们的研究结果表明,RecA 核蛋白丝能够在三种不同的机械状态之间连续循环,这可能对 RecA 介导的 HR 过程具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号