当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding of inhibitors to active-site mutants of CD1, the enigmatic catalytic domain of histone deacetylase 6.
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-09-03 , DOI: 10.1107/s2053230x20010250 Jeremy D Osko 1 , David W Christianson 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-09-03 , DOI: 10.1107/s2053230x20010250 Jeremy D Osko 1 , David W Christianson 1
Affiliation
|
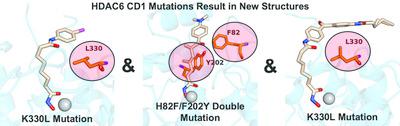
The zinc hydrolase histone deacetylase 6 (HDAC6) is unique among vertebrate deacetylases in that it contains two catalytic domains, designated CD1 and CD2. Both domains are fully functional as lysine deacetylases in vitro. However, the in vivo function of only the CD2 domain is well defined, whereas that of the CD1 domain is more enigmatic. Three X‐ray crystal structures of HDAC6 CD1–inhibitor complexes are now reported to broaden the understanding of affinity determinants in the active site. Notably, cocrystallization with inhibitors was facilitated by using active‐site mutants of zebrafish HDAC6 CD1. The first mutant studied, H82F/F202Y HDAC6 CD1, was designed to mimic the active site of human HDAC6 CD1. The structure of its complex with trichostatin A was generally identical to that with the wild‐type zebrafish enzyme. The second mutant studied, K330L HDAC6 CD1, was prepared to mimic the active site of HDAC6 CD2. It has previously been demonstrated that this substitution does not perturb inhibitor binding conformations in HDAC6 CD1; here, this mutant facilitated cocrystallization with derivatives of the cancer chemotherapy drug suberoylanilide hydroxamic acid (SAHA). These crystal structures allow the mapping of inhibitor‐binding regions in the outer active‐site cleft, where one HDAC isozyme typically differs from another. It is expected that these structures will help to guide the structure‐based design of inhibitors with selectivity against HDAC6 CD1, which in turn will enable new chemical biology approaches to probe its cellular function.
中文翻译:

抑制剂与 CD1(组蛋白脱乙酰酶 6 的神秘催化结构域)活性位点突变体的结合。
锌水解酶组蛋白脱乙酰酶 6 (HDAC6) 在脊椎动物脱乙酰酶中是独特的,因为它包含两个催化结构域,分别称为 CD1 和 CD2。这两个结构域在体外都具有完整的赖氨酸脱乙酰酶功能。然而,只有 CD2 结构域的体内功能是明确的,而 CD1 结构域的体内功能则更加神秘。目前报道了 HDAC6 CD1 抑制剂复合物的三种 X 射线晶体结构,以拓宽对活性位点亲和力决定因素的理解。值得注意的是,通过使用斑马鱼 HDAC6 CD1 的活性位点突变体促进了与抑制剂的共结晶。研究的第一个突变体 H82F/F202Y HDAC6 CD1 旨在模拟人类 HDAC6 CD1 的活性位点。其与曲古抑菌素 A 的复合物结构与野生型斑马鱼酶的复合物结构基本相同。研究的第二个突变体 K330L HDAC6 CD1 是为了模拟 HDAC6 CD2 的活性位点而制备的。先前已证明这种取代不会扰乱 HDAC6 CD1 中的抑制剂结合构象;在此,该突变体促进了与癌症化疗药物辛二酰苯胺异羟肟酸(SAHA)衍生物的共结晶。这些晶体结构允许在外部活性位点裂缝中绘制抑制剂结合区域,其中一种 HDAC 同工酶通常与另一种不同。预计这些结构将有助于指导基于结构的 HDAC6 CD1 选择性抑制剂的设计,从而使新的化学生物学方法能够探测其细胞功能。
更新日期:2020-09-03
中文翻译:

抑制剂与 CD1(组蛋白脱乙酰酶 6 的神秘催化结构域)活性位点突变体的结合。
锌水解酶组蛋白脱乙酰酶 6 (HDAC6) 在脊椎动物脱乙酰酶中是独特的,因为它包含两个催化结构域,分别称为 CD1 和 CD2。这两个结构域在体外都具有完整的赖氨酸脱乙酰酶功能。然而,只有 CD2 结构域的体内功能是明确的,而 CD1 结构域的体内功能则更加神秘。目前报道了 HDAC6 CD1 抑制剂复合物的三种 X 射线晶体结构,以拓宽对活性位点亲和力决定因素的理解。值得注意的是,通过使用斑马鱼 HDAC6 CD1 的活性位点突变体促进了与抑制剂的共结晶。研究的第一个突变体 H82F/F202Y HDAC6 CD1 旨在模拟人类 HDAC6 CD1 的活性位点。其与曲古抑菌素 A 的复合物结构与野生型斑马鱼酶的复合物结构基本相同。研究的第二个突变体 K330L HDAC6 CD1 是为了模拟 HDAC6 CD2 的活性位点而制备的。先前已证明这种取代不会扰乱 HDAC6 CD1 中的抑制剂结合构象;在此,该突变体促进了与癌症化疗药物辛二酰苯胺异羟肟酸(SAHA)衍生物的共结晶。这些晶体结构允许在外部活性位点裂缝中绘制抑制剂结合区域,其中一种 HDAC 同工酶通常与另一种不同。预计这些结构将有助于指导基于结构的 HDAC6 CD1 选择性抑制剂的设计,从而使新的化学生物学方法能够探测其细胞功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号