当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption, Thermodynamic, and Experimental Studies of Corrosion Inhibitor of Tin in 0.2 M Maleic Acid by Hydrogen Phosphate Ions (HPO42−)
Electroanalysis ( IF 2.7 ) Pub Date : 2020-09-02 , DOI: 10.1002/elan.202060406 Brahim Ait Addi 1 , Abdelaziz Ait Addi 1 , Abdul Shaban 2 , El‐Habib Ait Addi 3 , Mohamed Hamdani 1
Electroanalysis ( IF 2.7 ) Pub Date : 2020-09-02 , DOI: 10.1002/elan.202060406 Brahim Ait Addi 1 , Abdelaziz Ait Addi 1 , Abdul Shaban 2 , El‐Habib Ait Addi 3 , Mohamed Hamdani 1
Affiliation
|
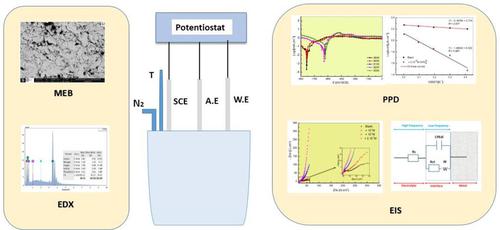
The inhibition efficiency of H2PO42− ions against tin corrosion in 0.2 M maleic acid is studied using electrochemical methods, surface analytical methods, and thermodynamic analysis. The potentiodynamic polarization plots showed the presence of an active/passive transition state of the tin electrode. The EIS measurements confirmed that the inhibition efficiency of H2PO42− increased by increasing the concentration (η=81 % at Cinh=2.10−2 M) and decreased by rising the temperature. The polarization tests demonstrated that the inhibitor performs as a cathodic‐type. The adsorption of the inhibitor was spontaneous and followed the Langmuir adsorption isotherm. A model of the inhibition mechanism was suggested.
中文翻译:

磷酸氢根离子(HPO42-)在0.2 M马来酸中锡对锡的吸附,热力学和实验研究
利用电化学方法,表面分析方法和热力学分析研究了H 2 PO 4 2-离子对0.2 M马来酸中锡腐蚀的抑制作用。电位动力学极化图显示了锡电极存在主动/被动过渡态。EIS测量结果证实,H 2 PO 4 2-的抑制效率通过增加浓度而增加(在C inh = 2.10 -2时, η= 81% M),并通过升高温度降低。极化测试表明该抑制剂表现为阴极型。抑制剂的吸附是自发的,并且遵循Langmuir吸附等温线。提出了抑制机制的模型。
更新日期:2020-09-02
中文翻译:

磷酸氢根离子(HPO42-)在0.2 M马来酸中锡对锡的吸附,热力学和实验研究
利用电化学方法,表面分析方法和热力学分析研究了H 2 PO 4 2-离子对0.2 M马来酸中锡腐蚀的抑制作用。电位动力学极化图显示了锡电极存在主动/被动过渡态。EIS测量结果证实,H 2 PO 4 2-的抑制效率通过增加浓度而增加(在C inh = 2.10 -2时, η= 81% M),并通过升高温度降低。极化测试表明该抑制剂表现为阴极型。抑制剂的吸附是自发的,并且遵循Langmuir吸附等温线。提出了抑制机制的模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号