当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gut microbiome alterations in type 1 autoimmune pancreatitis after induction of remission by prednisolone.
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-09-02 , DOI: 10.1111/cei.13509 K Kamata 1 , T Watanabe 1 , K Minaga 1 , A Hara 1 , I Sekai 1 , Y Otsuka 1 , T Yoshikawa 1 , A-M Park 2 , M Kudo 1
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-09-02 , DOI: 10.1111/cei.13509 K Kamata 1 , T Watanabe 1 , K Minaga 1 , A Hara 1 , I Sekai 1 , Y Otsuka 1 , T Yoshikawa 1 , A-M Park 2 , M Kudo 1
Affiliation
|
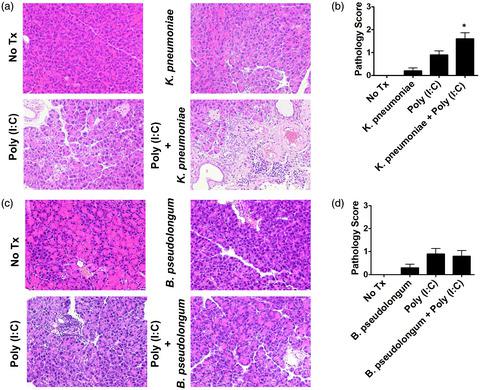
Although increasing evidence demonstrates the association between intestinal dysbiosis and pancreatic diseases such as chronic pancreatitis and pancreatic cancer, it remains largely unknown whether intestinal dysbiosis is involved in the immunopathogenesis of autoimmune pancreatitis (AIP). Recently, we found that intestinal dysbiosis mediates experimental AIP via the activation of plasmacytoid dendritic cells (pDCs), which can produce interferon (IFN)‐α and interleukin (IL)‐33. However, candidate intestinal bacteria, which promote the development of AIP, have not been identified. Fecal samples were obtained from type 1 AIP patients before and after prednisolone (PSL) treatment and subjected to 16S ribosomal RNA sequencing to evaluate the composition of intestinal bacteria. Induction of remission by PSL was associated with the complete disappearance of Klebsiella species from feces in two of the three analyzed patients with type 1 AIP. To assess the pathogenicity of Klebsiella species, mild experimental AIP was induced in MRL/MpJ mice by repeated injections of 10 μg of polyinosinic–polycytidylic acid [poly(I:C)], in combination with oral administration of heat‐killed Klebsiella pneumoniae. The AIP pathology score was significantly higher in MRL/MpJ mice that received both oral administration of heat‐killed K. pneumoniae and intraperitoneal injections of poly(I:C) than in those administered either agent alone. Pancreatic accumulation of pDCs capable of producing large amounts of IFN‐α and IL‐33 was also significantly higher in mice that received both treatments. These data suggest that intestinal colonization by K. pneumoniae may play an intensifying role in the development of type 1 AIP.
中文翻译:

泼尼松龙诱导缓解后 1 型自身免疫性胰腺炎的肠道微生物组改变。
尽管越来越多的证据表明肠道菌群失调与慢性胰腺炎和胰腺癌等胰腺疾病之间存在关联,但肠道菌群失调是否与自身免疫性胰腺炎 (AIP) 的免疫发病机制有关仍然未知。最近,我们发现肠道生态失调通过激活浆细胞样树突细胞 (pDC) 介导实验性 AIP,pDC 可以产生干扰素 (IFN)-α 和白细胞介素 (IL)-33。然而,尚未确定促进 AIP 发展的候选肠道细菌。在泼尼松龙 (PSL) 治疗前后从 1 型 AIP 患者中获取粪便样本,并进行 16S 核糖体 RNA 测序以评估肠道细菌的组成。来自三个分析的 1 型 AIP 患者中的两个的粪便中的克雷伯氏菌。为了评估克雷伯菌属物种的致病性,通过重复注射 10 μg 聚肌苷-聚胞苷酸 [poly(I:C)],并结合口服热灭活的肺炎克雷伯菌,在 MRL/MpJ 小鼠中诱导温和的实验性 AIP 。AIP 病理学评分在 MRL/MpJ 小鼠中显着更高,同时接受了热灭活肺炎克雷伯菌的口服给药和腹膜内注射聚 (I:C) 比单独给药的那些。在接受两种治疗的小鼠中,能够产生大量 IFN-α 和 IL-33 的 pDC 的胰腺积累也显着更高。这些数据表明,肺炎克雷伯菌在肠道的定植可能在 1 型 AIP 的发展中起强化作用。
更新日期:2020-09-02
中文翻译:

泼尼松龙诱导缓解后 1 型自身免疫性胰腺炎的肠道微生物组改变。
尽管越来越多的证据表明肠道菌群失调与慢性胰腺炎和胰腺癌等胰腺疾病之间存在关联,但肠道菌群失调是否与自身免疫性胰腺炎 (AIP) 的免疫发病机制有关仍然未知。最近,我们发现肠道生态失调通过激活浆细胞样树突细胞 (pDC) 介导实验性 AIP,pDC 可以产生干扰素 (IFN)-α 和白细胞介素 (IL)-33。然而,尚未确定促进 AIP 发展的候选肠道细菌。在泼尼松龙 (PSL) 治疗前后从 1 型 AIP 患者中获取粪便样本,并进行 16S 核糖体 RNA 测序以评估肠道细菌的组成。来自三个分析的 1 型 AIP 患者中的两个的粪便中的克雷伯氏菌。为了评估克雷伯菌属物种的致病性,通过重复注射 10 μg 聚肌苷-聚胞苷酸 [poly(I:C)],并结合口服热灭活的肺炎克雷伯菌,在 MRL/MpJ 小鼠中诱导温和的实验性 AIP 。AIP 病理学评分在 MRL/MpJ 小鼠中显着更高,同时接受了热灭活肺炎克雷伯菌的口服给药和腹膜内注射聚 (I:C) 比单独给药的那些。在接受两种治疗的小鼠中,能够产生大量 IFN-α 和 IL-33 的 pDC 的胰腺积累也显着更高。这些数据表明,肺炎克雷伯菌在肠道的定植可能在 1 型 AIP 的发展中起强化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号