Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ubiquitin-Modulated Phase Separation of Shuttle Proteins: Does Condensate Formation Promote Protein Degradation?
BioEssays ( IF 3.2 ) Pub Date : 2020-09-03 , DOI: 10.1002/bies.202000036 Thuy P Dao 1 , Carlos A Castañeda 1, 2, 3
BioEssays ( IF 3.2 ) Pub Date : 2020-09-03 , DOI: 10.1002/bies.202000036 Thuy P Dao 1 , Carlos A Castañeda 1, 2, 3
Affiliation
|
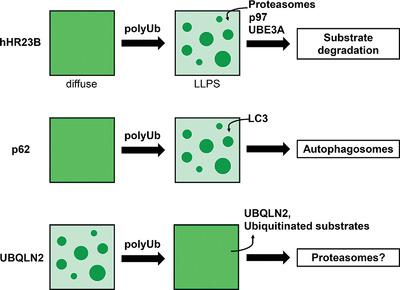
Liquid‐liquid phase separation (LLPS) has recently emerged as a possible mechanism that enables ubiquitin‐binding shuttle proteins to facilitate the degradation of ubiquitinated substrates via distinct protein quality control (PQC) pathways. Shuttle protein LLPS is modulated by multivalent interactions among their various domains as well as heterotypic interactions with polyubiquitin chains. Here, the properties of three different shuttle proteins (hHR23B, p62, and UBQLN2) are closely examined, unifying principles for the molecular determinants of their LLPS are identified, and how LLPS is connected to their functions is discussed. Evidence supporting LLPS of other shuttle proteins is also found. In this review, it is proposed that shuttle protein LLPS leads to spatiotemporal regulation of PQC activities by mediating the recruitment of PQC machinery (including proteasomes or autophagic components) to biomolecular condensates, assembly/disassembly of condensates, selective enrichment of client proteins, and extraction of ubiquitinated proteins from condensates in cells.
中文翻译:

泛素调节的穿梭蛋白相分离:冷凝物的形成是否促进蛋白质降解?
液-液相分离(LLPS)最近成为一种可能的机制,使泛素结合穿梭蛋白能够通过不同的蛋白质质量控制(PQC)途径促进泛素化底物的降解。穿梭蛋白 LLPS 通过其各个结构域之间的多价相互作用以及与多聚泛素链的异型相互作用进行调节。在这里,我们仔细研究了三种不同穿梭蛋白(hHR23B、p62 和 UBQLN2)的特性,确定了其 LLPS 分子决定因素的统一原理,并讨论了 LLPS 如何与其功能相关。还发现了支持其他穿梭蛋白 LLPS 的证据。在这篇综述中,提出穿梭蛋白 LLPS 通过介导 PQC 机制(包括蛋白酶体或自噬成分)向生物分子凝聚物的募集、凝聚物的组装/分解、客户蛋白的选择性富集和提取,从而导致 PQC 活动的时空调节。来自细胞内浓缩物的泛素化蛋白质。
更新日期:2020-10-22
中文翻译:

泛素调节的穿梭蛋白相分离:冷凝物的形成是否促进蛋白质降解?
液-液相分离(LLPS)最近成为一种可能的机制,使泛素结合穿梭蛋白能够通过不同的蛋白质质量控制(PQC)途径促进泛素化底物的降解。穿梭蛋白 LLPS 通过其各个结构域之间的多价相互作用以及与多聚泛素链的异型相互作用进行调节。在这里,我们仔细研究了三种不同穿梭蛋白(hHR23B、p62 和 UBQLN2)的特性,确定了其 LLPS 分子决定因素的统一原理,并讨论了 LLPS 如何与其功能相关。还发现了支持其他穿梭蛋白 LLPS 的证据。在这篇综述中,提出穿梭蛋白 LLPS 通过介导 PQC 机制(包括蛋白酶体或自噬成分)向生物分子凝聚物的募集、凝聚物的组装/分解、客户蛋白的选择性富集和提取,从而导致 PQC 活动的时空调节。来自细胞内浓缩物的泛素化蛋白质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号