Tetrahedron ( IF 2.1 ) Pub Date : 2020-09-03 , DOI: 10.1016/j.tet.2020.131503 Kei Yanai , Hideo Togo
|
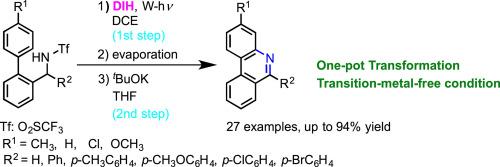
Treatment of N-(o-arylbenzyl)trifluoromethanesulfonamides with 1,3-diiodo-5,5-dimethylhydantoin in 1,2-dichloroethane under irradiation with a tungsten lamp, followed by the reaction with tBuOK gave the corresponding 6-arylphenanthridines and 6-unsubstituted phenanthridines in good to moderate yields, respectively. The reaction proceeds through an oxidative cyclization onto the aromatic ring by sulfonamidyl radicals formed from the N-iodosulfonamides. The present reaction is a one-pot method for the preparation of both 6-arylphenanthridines and 6-unsubstituted phenanthridines from N-(o-arylbenzyl)trifluoromethanesulfonamides under transition-metal-free conditions. © 2020 Elsevier Science. All rights reserved.
中文翻译:

由N-(邻芳基苄基)三氟甲烷磺酰胺与1,3-二碘-5,5-二甲基乙内酰脲制备菲啶
在钨灯的照射下,在1,2-二氯乙烷中用1,3-二碘-5,5-二甲基乙内酰脲处理N-(邻芳基苄基)三氟甲磺酰胺,然后与t BuOK反应,得到相应的6-芳基菲啶和6 -未取代的菲啶分别以良好至中等的产率。该反应通过由N-碘磺酰胺形成的磺酰胺基,通过氧化环化反应进行到芳环上。本反应是一锅法,可从N-(o)制备6-芳基菲啶和6-未取代的菲啶-芳基苄基)三氟甲磺酰胺在无过渡金属的条件下。©2020爱思唯尔科学。版权所有。











































 京公网安备 11010802027423号
京公网安备 11010802027423号