当前位置:
X-MOL 学术
›
Enzyme Microb. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Production and characterisation of a novel actinobacterial DyP-type peroxidase and its application in coupling of phenolic monomers
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.enzmictec.2020.109654 Amos Musengi , Kim Durrell , Alaric Prins , Nuraan Khan , Mayowa Agunbiade , Tukayi Kudanga , Bronwyn Kirby-McCullough , Brett I. Pletschke , Stephanie G. Burton , Marilize Le Roes-Hill
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.enzmictec.2020.109654 Amos Musengi , Kim Durrell , Alaric Prins , Nuraan Khan , Mayowa Agunbiade , Tukayi Kudanga , Bronwyn Kirby-McCullough , Brett I. Pletschke , Stephanie G. Burton , Marilize Le Roes-Hill
|
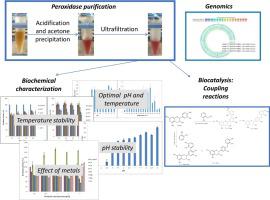
The extracellular peroxidase from Streptomyces albidoflavus BSII#1 was purified to near homogeneity using sequential steps of acid and acetone precipitation, followed by ultrafiltration. The purified peroxidase was characterised and tested for the ability to catalyse coupling reactions between selected phenolic monomer pairs. A 46-fold purification of the peroxidase was achieved, and it was shown to be a 46 kDa haem peroxidase. Unlike other actinobacteria-derived peroxidases, it was only inhibited (27 % inhibition) by relatively high concentrations of sodium azide (5 mM) and was capable of oxidising eleven (2,4-dichlorophenol, 2,6-dimethoxyphenol, 4-tert-butylcatechol, ABTS, caffeic acid, catechol, guaiacol, l-DOPA, o-aminophenol, phenol, pyrogallol) of the seventeen substrates tested. The peroxidase remained stable at temperatures of up to 80 °C for 60 min and retained >50 % activity after 24 h between pH 5.0-9.0, but was most sensitive to incubation with hydrogen peroxide (H2O2; 0.01 mM), l-cysteine (0.02 mM) and ascorbate (0.05 mM) for one hour. It was significantly inhibited by all organic solvents tested (p ≤ 0.05). The Km and Vmax values of the partially purified peroxidase with the substrate 2,4-DCP were 0.95 mM and 0.12 mmol min-1, respectively. The dyes reactive blue 4, reactive black 5, and Azure B, were all decolourised to a certain extent: approximately 30 % decolourisation was observed after 24 h (1 μM dye). The peroxidase successfully catalysed coupling reactions between several phenolic monomer pairs including catechin-caffeic acid, catechin-catechol, catechin-guaiacol and guaiacol-syringaldazine under the non-optimised conditions used in this study. Genome sequencing confirmed the identity of strain BSII#1 as a S. albidoflavus strain. In addition, the genome sequence revealed the presence of one peroxidase gene that includes the twin arginine translocation signal sequence of extracellular proteins. Functional studies confirmed that the peroxidase produced by S. albidoflavus BSII#1 is part of the dye-decolourising peroxidase (DyP-type) family.
中文翻译:

一种新型放线菌DyP型过氧化物酶的制备与表征及其在酚类单体偶联中的应用
来自白色链霉菌 BSII#1 的细胞外过氧化物酶使用酸和丙酮沉淀的连续步骤纯化至接近同质,然后超滤。对纯化的过氧化物酶进行表征并测试其催化所选酚单体对之间偶联反应的能力。实现了过氧化物酶的 46 倍纯化,它显示为 46 kDa 血红素过氧化物酶。与其他放线菌来源的过氧化物酶不同,它仅被相对高浓度的叠氮化钠 (5 mM) 抑制(27 % 抑制),并且能够氧化 11 种(2,4-二氯苯酚、2,6-二甲氧基苯酚、4-叔-丁基儿茶酚、ABTS、咖啡酸、儿茶酚、愈创木酚、l-多巴、邻氨基苯酚、苯酚、连苯三酚)的 17 种测试底物。过氧化物酶在高达 80 °C 的温度下保持稳定 60 分钟,并在 pH 5.0-9.0 之间 24 小时后保持 >50% 的活性,但对与过氧化氢(H2O2;0.01 mM)、l-半胱氨酸孵育最敏感( 0.02 mM) 和抗坏血酸 (0.05 mM) 一小时。它被所有测试的有机溶剂显着抑制 (p ≤ 0.05)。带有底物 2,4-DCP 的部分纯化过氧化物酶的 Km 和 Vmax 值分别为 0.95 mM 和 0.12 mmol min-1。染料活性蓝 4、活性黑 5 和 Azure B 都在一定程度上脱色:24 小时后观察到大约 30% 的脱色(1 μM 染料)。过氧化物酶成功地催化了几种酚单体对之间的偶联反应,包括儿茶素-咖啡酸、儿茶素-儿茶酚、本研究中使用的非优化条件下的儿茶素-愈创木酚和愈创木酚-丁香醛连氮。基因组测序证实菌株 BSII#1 为白色链球菌菌株。此外,基因组序列揭示了一种过氧化物酶基因的存在,该基因包括胞外蛋白的双精氨酸易位信号序列。功能研究证实,白黄链球菌 BSII#1 产生的过氧化物酶是染料脱色过氧化物酶(DyP 型)家族的一部分。
更新日期:2020-11-01
中文翻译:

一种新型放线菌DyP型过氧化物酶的制备与表征及其在酚类单体偶联中的应用
来自白色链霉菌 BSII#1 的细胞外过氧化物酶使用酸和丙酮沉淀的连续步骤纯化至接近同质,然后超滤。对纯化的过氧化物酶进行表征并测试其催化所选酚单体对之间偶联反应的能力。实现了过氧化物酶的 46 倍纯化,它显示为 46 kDa 血红素过氧化物酶。与其他放线菌来源的过氧化物酶不同,它仅被相对高浓度的叠氮化钠 (5 mM) 抑制(27 % 抑制),并且能够氧化 11 种(2,4-二氯苯酚、2,6-二甲氧基苯酚、4-叔-丁基儿茶酚、ABTS、咖啡酸、儿茶酚、愈创木酚、l-多巴、邻氨基苯酚、苯酚、连苯三酚)的 17 种测试底物。过氧化物酶在高达 80 °C 的温度下保持稳定 60 分钟,并在 pH 5.0-9.0 之间 24 小时后保持 >50% 的活性,但对与过氧化氢(H2O2;0.01 mM)、l-半胱氨酸孵育最敏感( 0.02 mM) 和抗坏血酸 (0.05 mM) 一小时。它被所有测试的有机溶剂显着抑制 (p ≤ 0.05)。带有底物 2,4-DCP 的部分纯化过氧化物酶的 Km 和 Vmax 值分别为 0.95 mM 和 0.12 mmol min-1。染料活性蓝 4、活性黑 5 和 Azure B 都在一定程度上脱色:24 小时后观察到大约 30% 的脱色(1 μM 染料)。过氧化物酶成功地催化了几种酚单体对之间的偶联反应,包括儿茶素-咖啡酸、儿茶素-儿茶酚、本研究中使用的非优化条件下的儿茶素-愈创木酚和愈创木酚-丁香醛连氮。基因组测序证实菌株 BSII#1 为白色链球菌菌株。此外,基因组序列揭示了一种过氧化物酶基因的存在,该基因包括胞外蛋白的双精氨酸易位信号序列。功能研究证实,白黄链球菌 BSII#1 产生的过氧化物酶是染料脱色过氧化物酶(DyP 型)家族的一部分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号