Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-09-03 , DOI: 10.1016/j.bioorg.2020.104261 Xiaofeng Zheng 1 , Abdukriem Kadir 2 , Guijuan Zheng 1 , Pengfei Jin 1 , Dongmei Qin 3 , Maitinuer Maiwulanjiang 4 , Haji Akber Aisa 4 , Guangmin Yao 1
|
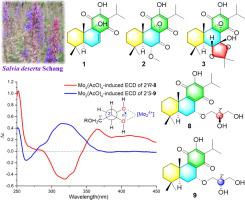
A total of twenty abietane quinone diterpenoids including ten new ones (1–10) were isolated from the roots extract of Salvia deserta. Their chemical structures were delineated by extensive spectrometric and spectroscopic techniques including HRESIMS, NMR, UV, IR, and single-crystal X-ray diffraction analysis, calculated 13C NMR-DP4+ analysis, calculated ECD, and Mo2(OAc)4-induced ECD. The absolute configurations of salvidesertone A (1), 8α,9α-epoxy-6-deoxycoleon U (18), and 7,20-epoxyroyleanone (19) were determined by single-crystal X-ray diffraction analysis. Salvidesertone A (1) represents the first example of a 9-hydroxyabieta-7(8)-ene quinone diterpenoid. This is the first report of the crystal structures of 8α,9α-epoxy-6-deoxycoleon U (18) and 7,20-epoxyroyleanone (19). Abietane quinone diterpenoids 1, 2, and 4–20 were evaluated for their antiproliferative activities against five cancer cell lines A-549, SMMC-7721, SW480, MCF-7, and HL-60 and a normal epithelial cell line BEAS-2B in vitro. Salvidesertones E (8) and F (9) selectively inhibited the proliferation of A-549, SMMC-7721, and SW480 cancer cell lines. Importantly, salvidesertones E (8) and F (9), horminone (13), taxoquinone (14), 7α-O-methylhorminone (15), and 8α,9α-epoxy-6-deoxycoleon U (18) showed more potent antiproliferative effects against A-549 than the positive control cis-platin. A preliminary structure–activity relationship for the antiproliferative effects of abietane quinone diterpenoids 1–20 was discussed.











































 京公网安备 11010802027423号
京公网安备 11010802027423号