当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remote C(sp3)–H vinylation via radical-mediated consecutive fission of C–H and C–C bonds
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0qo00952k Tao Niu 1, 2, 3, 4, 5 , Shan Yang 1, 2, 3, 4, 5 , Xinxin Wu 1, 2, 3, 4, 5 , Chen Zhu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0qo00952k Tao Niu 1, 2, 3, 4, 5 , Shan Yang 1, 2, 3, 4, 5 , Xinxin Wu 1, 2, 3, 4, 5 , Chen Zhu 1, 2, 3, 4, 5
Affiliation
|
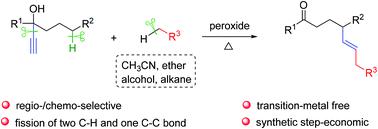
Described herein is a radical-mediated vinylation of the remote C(sp3)–H bonds of propargylic alcohols. A variety of widely available reagents, such as acetonitrile, ethers, aliphatic alcohols, and simple alkanes, serve as both the solvent and coupling partner. The transformation proceeds via sequential vinyl radical-mediated HAT and intramolecular vinyl migration, in which two inert C(sp3)–H bonds and one C–C bond are homolysed. The reaction also features broad functional group tolerance, good regio-/chemo-selectivity, and synthetic step-economy.
中文翻译:

通过自由基介导的C–H和C–C键的连续裂变实现远程C(sp3)–H乙烯基化
本文描述的是炔丙醇的远端C(sp 3)-H键的自由基介导的乙烯基化。多种广泛使用的试剂,例如乙腈,醚,脂族醇和简单的烷烃,既可以作为溶剂,也可以作为偶联剂。转化过程是通过顺序的乙烯基自由基介导的HAT和分子内的乙烯基迁移进行的,其中两个惰性C(sp 3)-H键和一个C-C键被均化。该反应还具有宽泛的官能团耐受性,良好的区域/化学选择性和合成步骤经济性。
更新日期:2020-09-30
中文翻译:

通过自由基介导的C–H和C–C键的连续裂变实现远程C(sp3)–H乙烯基化
本文描述的是炔丙醇的远端C(sp 3)-H键的自由基介导的乙烯基化。多种广泛使用的试剂,例如乙腈,醚,脂族醇和简单的烷烃,既可以作为溶剂,也可以作为偶联剂。转化过程是通过顺序的乙烯基自由基介导的HAT和分子内的乙烯基迁移进行的,其中两个惰性C(sp 3)-H键和一个C-C键被均化。该反应还具有宽泛的官能团耐受性,良好的区域/化学选择性和合成步骤经济性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号