当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-bromo- and 2-phenyl-neo-confused porphyrins.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0ob01642j Arwa S Almejbel 1 , Timothy D Lash 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0ob01642j Arwa S Almejbel 1 , Timothy D Lash 1
Affiliation
|
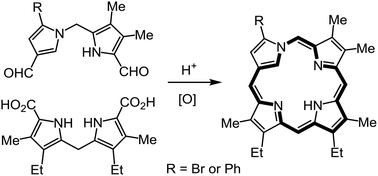
Neo-confused porphyrins (neo-CPs), porphyrin isomers with a 1,3-connected pyrrolic subunit, are aromatic structures with a CNNN coordination core. Previously, examples of neo-CPs with fused benzo units or electron-withdrawing ester substituents have been described. In this paper, two new examples of neo-CPs are reported that lack a fused aromatic unit or an ester moiety, but instead have a bromo or phenyl substituent on the neo-confused ring. Acid-catalyzed condensation of suitably substituted 1,2′-dipyrrylmethane dialdehydes with a 2,2′-dipyrrylmethane, followed by oxidation with aqueous ferric chloride solutions, afforded the neo-CPs in 40–45% yield. These porphyrin analogues had slightly reduced diatropic ring currents and slowly decomposed in solution. The related palladium(II) and nickel(II) complexes proved to be very unstable, even though the diatropicity of the macrocycle was enhanced. This study shows that stabilizing substituents are necessary for investigations into this class of porphyrinoids. Attempts to prepare imidazole versions of neo-CPs were unsuccessful.
中文翻译:

2-溴和 2-苯基-新混淆卟啉的合成。
Neo-confused porphyrins (neo-CPs) 是具有 1,3 连接的吡咯亚基的卟啉异构体,是具有 CNNN 配位核心的芳香结构。以前,已经描述了具有稠合苯并单元或吸电子酯取代基的新 CP 的例子。在本文中,报告了两个新的新 CP 实例,它们缺少稠合芳族单元或酯部分,而是在新稠合环上具有溴或苯基取代基。适当取代的 1,2'-二吡咯甲烷二醛与 2,2'-二吡咯甲烷的酸催化缩合,然后用氯化铁水溶液氧化,以 40-45% 的产率提供新 CP。这些卟啉类似物具有略微降低的二向环电流并在溶液中缓慢分解。相关的钯(Ⅱ)和镍(Ⅱ) 配合物被证明是非常不稳定的,即使大环的二向性增强了。该研究表明,稳定取代基对于研究这类卟啉类化合物是必要的。制备新 CP 的咪唑版本的尝试未成功。
更新日期:2020-09-30
中文翻译:

2-溴和 2-苯基-新混淆卟啉的合成。
Neo-confused porphyrins (neo-CPs) 是具有 1,3 连接的吡咯亚基的卟啉异构体,是具有 CNNN 配位核心的芳香结构。以前,已经描述了具有稠合苯并单元或吸电子酯取代基的新 CP 的例子。在本文中,报告了两个新的新 CP 实例,它们缺少稠合芳族单元或酯部分,而是在新稠合环上具有溴或苯基取代基。适当取代的 1,2'-二吡咯甲烷二醛与 2,2'-二吡咯甲烷的酸催化缩合,然后用氯化铁水溶液氧化,以 40-45% 的产率提供新 CP。这些卟啉类似物具有略微降低的二向环电流并在溶液中缓慢分解。相关的钯(Ⅱ)和镍(Ⅱ) 配合物被证明是非常不稳定的,即使大环的二向性增强了。该研究表明,稳定取代基对于研究这类卟啉类化合物是必要的。制备新 CP 的咪唑版本的尝试未成功。











































 京公网安备 11010802027423号
京公网安备 11010802027423号