当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TMSOTf-catalyzed synthesis of substituted quinazolines using hexamethyldisilazane as a nitrogen source under neat and microwave irradiation conditions.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0ob01507e Chieh-Kai Chan,Chien-Yu Lai,Cheng-Chung Wang
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0ob01507e Chieh-Kai Chan,Chien-Yu Lai,Cheng-Chung Wang
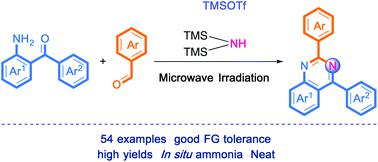
|
In this article, we report an efficient and mild synthetic route for the construction of substituted quinazolines from functionalized 2-aminobenzophenones with various benzaldehydes using cat. TMSOTf and hexamethyldisilazane (HMDS) under neat, metal-free and microwave irradiation conditions in which gaseous ammonia was formed in situ. This synthetic protocol provided the desired quinazolines with a broad substrate scope in good to excellent yields. Some structures were confirmed by X-ray single-crystal diffraction analysis.
中文翻译:

TMSOTf 催化合成取代喹唑啉,使用六甲基二硅氮烷作为氮源在纯和微波辐照条件下。
在本文中,我们报道了一种高效且温和的合成路线,用于使用cat由官能化的 2-氨基二苯甲酮与各种苯甲醛构建取代的喹唑啉。TMSOTf 和六甲基二硅氮烷 (HMDS) 在纯净、无金属和微波辐照条件下原位形成气态氨。这种合成方案提供了所需的喹唑啉,其底物范围广泛,产率良好。通过 X 射线单晶衍射分析证实了一些结构。
更新日期:2020-09-23
中文翻译:

TMSOTf 催化合成取代喹唑啉,使用六甲基二硅氮烷作为氮源在纯和微波辐照条件下。
在本文中,我们报道了一种高效且温和的合成路线,用于使用cat由官能化的 2-氨基二苯甲酮与各种苯甲醛构建取代的喹唑啉。TMSOTf 和六甲基二硅氮烷 (HMDS) 在纯净、无金属和微波辐照条件下原位形成气态氨。这种合成方案提供了所需的喹唑啉,其底物范围广泛,产率良好。通过 X 射线单晶衍射分析证实了一些结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号