当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Order-disorder transitions of cytoplasmic N-termini in the mechanisms of P-type ATPases
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-09-01 , DOI: 10.1039/d0fd00040j Khondker R Hossain 1 , Daniel Clayton 1 , Sophia C Goodchild 2 , Alison Rodger 2 , Richard J Payne 1 , Flemming Cornelius 3 , Ronald J Clarke 1, 4
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-09-01 , DOI: 10.1039/d0fd00040j Khondker R Hossain 1 , Daniel Clayton 1 , Sophia C Goodchild 2 , Alison Rodger 2 , Richard J Payne 1 , Flemming Cornelius 3 , Ronald J Clarke 1, 4
Affiliation
|
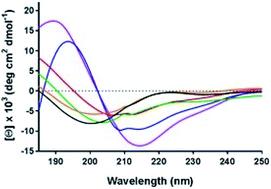
Membrane protein structure and function are modulated via interactions with their lipid environment. This is particularly true for integral membrane pumps, the P-type ATPases. These ATPases play vital roles in cell physiology, where they are associated with the transport of cations and lipids, thereby generating and maintaining crucial (electro-)chemical potential gradients across the membrane. Several pumps (Na+, K+-ATPase, H+, K+-ATPase and the plasma membrane Ca2+-ATPase) which are located in the asymmetric animal plasma membrane have been found to possess polybasic (lysine-rich) domains on their cytoplasmic surfaces, which are thought to act as phosphatidylserine (PS) binding domains. In contrast, the sarcoplasmic reticulum Ca2+-ATPase, located within an intracellular organelle membrane, does not possess such a domain. Here we focus on the lysine-rich N-termini of the plasma-membrane-bound Na+, K+- and H+, K+-ATPases. Synthetic peptides corresponding to the N-termini of these proteins were found, via quartz crystal microbalance and circular dichroism measurements, to interact via an electrostatic interaction with PS-containing membranes, thereby undergoing an increase in helical or other secondary structure content. As well as influencing ion pumping activity, it is proposed that this interaction could provide a mechanism for sensing the lipid asymmetry of the plasma membrane, which changes drastically when a cell undergoes apoptosis, i.e. programmed cell death. Thus, polybasic regions of plasma membrane-bound ion pumps could potentially perform the function of a “death sensor”, signalling to a cell to reduce pumping activity and save energy.
中文翻译:

P型ATP酶机制中细胞质N末端的有序-无序转变
膜蛋白的结构和功能通过与其脂质环境的相互作用进行调节。对于集成膜泵 P 型 ATP 酶来说尤其如此。这些 ATP 酶在细胞生理学中起着至关重要的作用,它们与阳离子和脂质的运输有关,从而产生和维持跨膜的关键(电)化学势梯度。几个泵(Na +、K + -ATPase、H +、K + -ATPase 和质膜 Ca 2+已发现位于不对称动物质膜中的-ATPase)在其细胞质表面上具有多元(富含赖氨酸)结构域,这些结构域被认为充当磷脂酰丝氨酸(PS)结合结构域。相反,位于细胞内细胞器膜内的肌浆网Ca 2+ -ATP酶不具有这样的结构域。在这里,我们关注质膜结合的 Na +、K + - 和 H +、K + -ATP酶的富含赖氨酸的 N 末端。通过石英晶体微量天平和圆二色性测量,发现与这些蛋白质的 N 末端相对应的合成肽通过以下方式相互作用与含 PS 的膜发生静电相互作用,从而增加螺旋或其他二级结构的含量。除了影响离子泵活性外,还提出这种相互作用可以提供一种机制来感知质膜的脂质不对称性,当细胞经历凋亡即程序性细胞死亡时,这种不对称性会发生巨大变化。因此,质膜结合离子泵的多碱基区域可能会发挥“死亡传感器”的功能,向细胞发出信号以减少泵送活动并节省能量。
更新日期:2020-09-01
中文翻译:

P型ATP酶机制中细胞质N末端的有序-无序转变
膜蛋白的结构和功能通过与其脂质环境的相互作用进行调节。对于集成膜泵 P 型 ATP 酶来说尤其如此。这些 ATP 酶在细胞生理学中起着至关重要的作用,它们与阳离子和脂质的运输有关,从而产生和维持跨膜的关键(电)化学势梯度。几个泵(Na +、K + -ATPase、H +、K + -ATPase 和质膜 Ca 2+已发现位于不对称动物质膜中的-ATPase)在其细胞质表面上具有多元(富含赖氨酸)结构域,这些结构域被认为充当磷脂酰丝氨酸(PS)结合结构域。相反,位于细胞内细胞器膜内的肌浆网Ca 2+ -ATP酶不具有这样的结构域。在这里,我们关注质膜结合的 Na +、K + - 和 H +、K + -ATP酶的富含赖氨酸的 N 末端。通过石英晶体微量天平和圆二色性测量,发现与这些蛋白质的 N 末端相对应的合成肽通过以下方式相互作用与含 PS 的膜发生静电相互作用,从而增加螺旋或其他二级结构的含量。除了影响离子泵活性外,还提出这种相互作用可以提供一种机制来感知质膜的脂质不对称性,当细胞经历凋亡即程序性细胞死亡时,这种不对称性会发生巨大变化。因此,质膜结合离子泵的多碱基区域可能会发挥“死亡传感器”的功能,向细胞发出信号以减少泵送活动并节省能量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号