当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to P-chiral sec- and tert-phosphine oxides enabled by Le-Phos-catalyzed asymmetric kinetic resolution
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0sc04041j Haile Qiu 1, 2, 3, 4 , Qiang Dai 1, 2, 3, 4 , Jiafeng He 1, 2, 3, 4 , Wenbo Li 1, 2, 3, 4 , Junliang Zhang 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0sc04041j Haile Qiu 1, 2, 3, 4 , Qiang Dai 1, 2, 3, 4 , Jiafeng He 1, 2, 3, 4 , Wenbo Li 1, 2, 3, 4 , Junliang Zhang 1, 2, 3, 4, 5
Affiliation
|
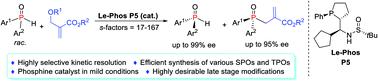
The synthesis of P-stereogenic building blocks is extremely difficult. Herein we report an efficient kinetic resolution of secondary phosphine oxides via a Le-Phos-catalyzed asymmetric allylation reaction with Morita–Baylis–Hillman carbonates. This method provides facile access to enantioenriched secondary and tertiary P-chiral phosphine oxides with broad substrate scope, both of which could serve as P-stereogenic synthons, and can be rapidly incorporated into a given scaffold bearing a P-stereocenter. The highly desirable late stage modifications demonstrate the practicability of our method and can be a critical contribution to obtaining optimal P-chiral catalysts and ligands.
中文翻译:

Le-Phos催化的不对称动力学拆分可实现对位手性仲和叔膦氧化物
P-立体异构结构单元的合成非常困难。在本文中,我们报告了通过Le-Phos催化与Morita–Baylis–Hillman碳酸盐的不对称烯丙基化反应,可以有效地动力学分解次膦氧化物。该方法提供了易于获得的,具有广泛底物范围的对映体富集的对映体和对映体的P-手性膦氧化物,两者均可以用作P-立体异构合成子,并且可以快速地掺入具有P-立体中心的给定支架中。高度期望的后期修饰证明了我们方法的实用性,并且可能对获得最佳的P-手性催化剂和配体起关键作用。
更新日期:2020-09-23
中文翻译:

Le-Phos催化的不对称动力学拆分可实现对位手性仲和叔膦氧化物
P-立体异构结构单元的合成非常困难。在本文中,我们报告了通过Le-Phos催化与Morita–Baylis–Hillman碳酸盐的不对称烯丙基化反应,可以有效地动力学分解次膦氧化物。该方法提供了易于获得的,具有广泛底物范围的对映体富集的对映体和对映体的P-手性膦氧化物,两者均可以用作P-立体异构合成子,并且可以快速地掺入具有P-立体中心的给定支架中。高度期望的后期修饰证明了我们方法的实用性,并且可能对获得最佳的P-手性催化剂和配体起关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号