当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Study of M[N(SO2F)(SO2CF3)]-[N-Butyl-N-methylpyrroridinium][N(SO2F)(SO2CF3)] (M = Li, Na, K, Rb, Cs) Ionic Liquid Electrolytes.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.jpcb.0c06578 Takayuki Yamamoto 1 , Shu Nishijima 1 , Toshiyuki Nohira 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.jpcb.0c06578 Takayuki Yamamoto 1 , Shu Nishijima 1 , Toshiyuki Nohira 1
Affiliation
|
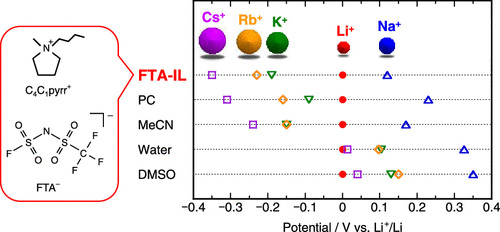
We systematically evaluated the physicochemical properties of a series of M[FTA]–[C4C1pyrr][FTA] ionic liquids (ILs) (M = alkali metal, FTA = (fluorosulfonyl)(trifluoromethylsulfonyl)amide, C4C1pyrr = N-butyl-N-methylpyrrolidinium) as electrolytes for alkali metal-ion batteries. First, the viscosity (η), ionic conductivity (σ), and density (ρ) of the M[FTA]–[C4C1pyrr][FTA] ILs at x(M[FTA]) = 0.20 (x(M[FTA]) = molar fraction of M[FTA]) were measured. The σ values ranged from 1–3 mS cm–1 at 298 K and increased as follows: Na < Li < K < Rb < Cs, which indicated that the Li-based IL did not obey the trend predicted by the charge densities of alkali metal cations. Second, the Li-based IL exhibited slightly lower vertical intercept values than the other FTA-based ILs in the Walden plots obtained using the results of η, σ, and ρ measurements. Third, the electrochemical stability of the ILs was investigated by cyclic voltammetry, and the redox potentials of the alkali metals (E(M+/M)) were determined. The E(M+/M) values of the FTA-based ILs increased as follows: Cs < Rb < K < Li < Na. Subsequently, we compared the obtained E(M+/M) values with those of other general electrolytes, such as propylene carbonate (PC)-based electrolytes and aqueous solutions. The trend in E(M+/M) values of the FTA-based ILs was similar to that of PC-based electrolytes and was significantly different from that of aqueous solutions. In particular, the FTA- and FSA-based ILs (FSA = bis(fluorosulfonyl)amide) presented the most negative E(Na+/Na) and E(K+/K) values among various electrolytes, which indicated that utilization of these IL electrolytes for the development of Na- and K-ion batteries would present significant advantages.
中文翻译:

M [N(SO2F)(SO2CF3)]-[N-丁基-N-甲基吡咯烷鎓] [N(SO2F)(SO2CF3)](M = Li,Na,K,Rb,Cs)离子液体电解质的比较研究。
我们系统地评估了一系列M [FTA] – [C 4 C 1 pyrr] [FTA]离子液体(ILs)的理化性质(M =碱金属,FTA =(氟磺酰基)(三氟甲基磺酰基)酰胺,C 4 C 1 pyrr = N-丁基-N-甲基吡咯烷鎓)作为碱金属离子电池的电解质。首先,在x(M [FTA])处M [FTA] – [C 4 C 1 pyrr] [FTA] ILs的粘度(η),离子电导率(σ)和密度(ρ )= 0.20(x( M [FTA])= M [FTA])的摩尔分数。σ值范围为1–3 mS cm –1在298 K时,Na <Li <K <Rb <Cs增大,这表明基于Li的IL没有遵循碱金属阳离子的电荷密度所预测的趋势。其次,在使用η,σ和ρ测量结果获得的Walden图中,基于Li的IL表现出比其他基于FTA的IL略低的垂直截距值。第三,通过循环伏安法研究了离子液体的电化学稳定性,并确定了碱金属的氧化还原电位(E(M + / M))。基于FTA的IL的E(M + / M)值增加如下:Cs <Rb <K <Li <Na。随后,我们比较了获得的E(M +/ M)值与其他一般电解质(例如,基于碳酸亚丙酯(PC)的电解质和水溶液)的值相同。基于FTA的IL的E(M + / M)值趋势类似于基于PC的电解质,并且与水溶液的趋势明显不同。尤其是,基于FTA和FSA的IL(FSA =双(氟磺酰基)酰胺)在各种电解质中呈现出最负的E(Na + / Na)和E(K + / K)值,这表明这些电解质的利用率用于开发Na和K离子电池的IL电解质将具有明显的优势。
更新日期:2020-09-24
中文翻译:

M [N(SO2F)(SO2CF3)]-[N-丁基-N-甲基吡咯烷鎓] [N(SO2F)(SO2CF3)](M = Li,Na,K,Rb,Cs)离子液体电解质的比较研究。
我们系统地评估了一系列M [FTA] – [C 4 C 1 pyrr] [FTA]离子液体(ILs)的理化性质(M =碱金属,FTA =(氟磺酰基)(三氟甲基磺酰基)酰胺,C 4 C 1 pyrr = N-丁基-N-甲基吡咯烷鎓)作为碱金属离子电池的电解质。首先,在x(M [FTA])处M [FTA] – [C 4 C 1 pyrr] [FTA] ILs的粘度(η),离子电导率(σ)和密度(ρ )= 0.20(x( M [FTA])= M [FTA])的摩尔分数。σ值范围为1–3 mS cm –1在298 K时,Na <Li <K <Rb <Cs增大,这表明基于Li的IL没有遵循碱金属阳离子的电荷密度所预测的趋势。其次,在使用η,σ和ρ测量结果获得的Walden图中,基于Li的IL表现出比其他基于FTA的IL略低的垂直截距值。第三,通过循环伏安法研究了离子液体的电化学稳定性,并确定了碱金属的氧化还原电位(E(M + / M))。基于FTA的IL的E(M + / M)值增加如下:Cs <Rb <K <Li <Na。随后,我们比较了获得的E(M +/ M)值与其他一般电解质(例如,基于碳酸亚丙酯(PC)的电解质和水溶液)的值相同。基于FTA的IL的E(M + / M)值趋势类似于基于PC的电解质,并且与水溶液的趋势明显不同。尤其是,基于FTA和FSA的IL(FSA =双(氟磺酰基)酰胺)在各种电解质中呈现出最负的E(Na + / Na)和E(K + / K)值,这表明这些电解质的利用率用于开发Na和K离子电池的IL电解质将具有明显的优势。









































 京公网安备 11010802027423号
京公网安备 11010802027423号