当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Aqueous Lower-Polarity Solvation Shells Around Amphiphilic 2,2,6,6-Tetramethylpiperidine-1-oxyl Radicals in Water.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1021/acs.jpcb.0c04863 Johannes Hunold 1 , Jana Eisermann 1 , Martin Brehm 1 , Dariush Hinderberger 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1021/acs.jpcb.0c04863 Johannes Hunold 1 , Jana Eisermann 1 , Martin Brehm 1 , Dariush Hinderberger 1
Affiliation
|
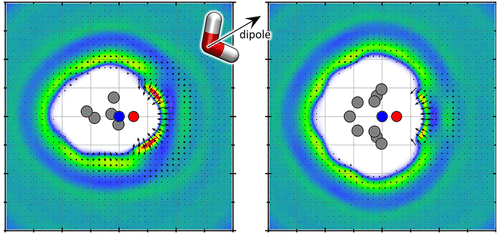
Solvation of the amphiphilic nitroxide radical 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and hydrophilic 4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPONE) in water and tetrahydrofuran (THF) is studied in detail. The existence of pure water shells enclosing TEMPO in an aqueous solution that leads to significantly reduced local polarity at the nitroxide moiety is shown with multifrequency electron paramagnetic resonance (EPR) spectroscopy at X- and Q-bands as well as spectral simulations. These aqueous lower-polarity solvation shells (ALPSS) offer TEMPO a local polarity that is similar to that in organic solvents like THF. Furthermore, using double electron–electron resonance spectroscopy, local enrichment and inhomogeneous distribution without direct molecular encounters of dissolved TEMPO in water are found that can be correlated with potentially attractive interactions mediated through ALPSS. However, no local enrichment of TEMPO is found in organic solvents such as THF. In contrast to TEMPO, the structurally very similar nitroxide radical TEMPONE shows no ALPSS encapsulation behavior with water molecules in aqueous solutions. Ensemble-averaging methods such as dynamic light scattering and electrospray ionization mass spectrometry substantiate the EPR spectroscopically obtained results of ALPSS-encased TEMPO and attractive interactions between them, leading to a higher local concentration. Furthermore, force field molecular dynamics simulations and metadynamics deliver support for our conclusions.
中文翻译:

水中两亲性2,2,6,6-四甲基哌啶-1-氧基自由基周围低极性溶剂化壳的表征。
两性硝基氧自由基2,2,6,6-四甲基哌啶-1-氧基(TEMPO)和亲水性4-oxo-2,2,6,6-四甲基哌啶-1-氧基(TEMPONE)在水和四氢呋喃(THF)中的溶解)进行了详细研究。通过X波段和Q波段的多频电子顺磁共振(EPR)光谱以及光谱模拟表明,存在将TEMPO封闭在水溶液中的纯水壳,该水溶液导致氮氧化物部分的局部极性大大降低。这些水性低极性溶剂化壳(ALPSS)为TEMPO提供的局部极性类似于THF等有机溶剂中的极性。此外,使用双电子-电子共振光谱,发现在水中没有溶解的TEMPO的直接分子相遇的局部富集和不均匀分布,这可能与通过ALPSS介导的潜在吸引相互作用有关。但是,在有机溶剂(如THF)中未发现TEMPO的局部富集。与TEMPO相反,在结构上非常相似的氮氧自由基TEMPONE在水溶液中对水分子无ALPSS包封行为。诸如动态光散射和电喷雾电离质谱之类的集合平均方法可证实EPR光谱学上获得的ALPSS包裹的TEMPO及其之间有吸引力的相互作用的结果,从而导致更高的局部浓度。此外,力场分子动力学模拟和元动力学为我们的结论提供了支持。
更新日期:2020-10-02
中文翻译:

水中两亲性2,2,6,6-四甲基哌啶-1-氧基自由基周围低极性溶剂化壳的表征。
两性硝基氧自由基2,2,6,6-四甲基哌啶-1-氧基(TEMPO)和亲水性4-oxo-2,2,6,6-四甲基哌啶-1-氧基(TEMPONE)在水和四氢呋喃(THF)中的溶解)进行了详细研究。通过X波段和Q波段的多频电子顺磁共振(EPR)光谱以及光谱模拟表明,存在将TEMPO封闭在水溶液中的纯水壳,该水溶液导致氮氧化物部分的局部极性大大降低。这些水性低极性溶剂化壳(ALPSS)为TEMPO提供的局部极性类似于THF等有机溶剂中的极性。此外,使用双电子-电子共振光谱,发现在水中没有溶解的TEMPO的直接分子相遇的局部富集和不均匀分布,这可能与通过ALPSS介导的潜在吸引相互作用有关。但是,在有机溶剂(如THF)中未发现TEMPO的局部富集。与TEMPO相反,在结构上非常相似的氮氧自由基TEMPONE在水溶液中对水分子无ALPSS包封行为。诸如动态光散射和电喷雾电离质谱之类的集合平均方法可证实EPR光谱学上获得的ALPSS包裹的TEMPO及其之间有吸引力的相互作用的结果,从而导致更高的局部浓度。此外,力场分子动力学模拟和元动力学为我们的结论提供了支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号