当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Reduction and Functionalization of Polycyclic Quinones.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02529 Karl J Thorley 1, 2 , Yang Song 3 , Sean R Parkin 1 , John E Anthony 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.orglett.0c02529 Karl J Thorley 1, 2 , Yang Song 3 , Sean R Parkin 1 , John E Anthony 1, 2
Affiliation
|
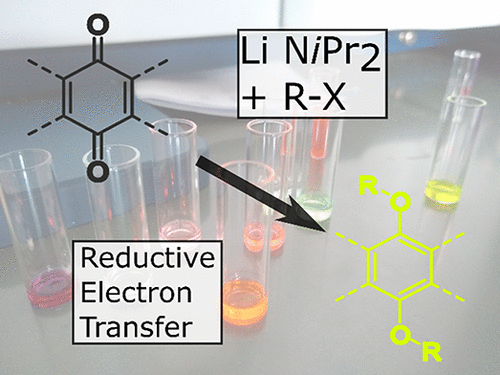
Attempts to functionalize polycyclic quinones using lithium diisopropylamide as a base led to the unexpected formation of acenes. This reaction proceeds by electron transfer from the base to the electron deficient quinone, whose radical anion can react with a variety of electrophiles. Siloxy derivatives synthesized by this method could be easily isolated but showed poor photostability. In situ reduced intermediate generation is a convenient approach to functionalization of oxidatively unstable hydroquinones.
中文翻译:

多环醌的原位还原和功能化。
尝试使用二异丙基氨基化锂作为碱将多环醌官能化会导致意外的乙炔形成。该反应通过电子从碱转移到缺电子的醌而进行,后者的自由基阴离子可与多种亲电子试剂反应。用这种方法合成的甲硅烷氧基衍生物很容易分离,但光稳定性差。原位减少的中间产物是氧化不稳定的氢醌功能化的简便方法。
更新日期:2020-09-20
中文翻译:

多环醌的原位还原和功能化。
尝试使用二异丙基氨基化锂作为碱将多环醌官能化会导致意外的乙炔形成。该反应通过电子从碱转移到缺电子的醌而进行,后者的自由基阴离子可与多种亲电子试剂反应。用这种方法合成的甲硅烷氧基衍生物很容易分离,但光稳定性差。原位减少的中间产物是氧化不稳定的氢醌功能化的简便方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号