当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Visible and Near-Infrared Light Activatable Diazo-Coumarin Probe for Fluorogenic Protein Labeling in Living Cells
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c08068 Sheng-Yao Dai 1 , Dan Yang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c08068 Sheng-Yao Dai 1 , Dan Yang 1
Affiliation
|
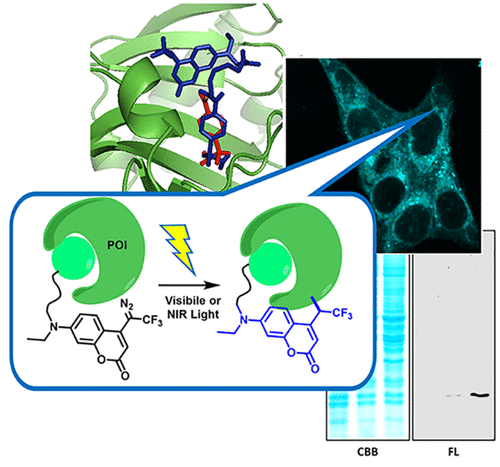
Chemical modification of proteins in living cells permits valuable glimpses into the molecular interactions that underpin dynamic cellular events. While genetic engineering methods are often preferred, selective labeling of endogenous proteins in a complex intracellular milieu with chemical approaches represents a significant challenge. In this study, we report novel diazo-coumarin compounds that can be photo-activated by visible (430‒490 nm) and near-infrared light (800 nm) irradiation to photo-uncage reactive carbene intermediates, which could subsequently undergo insertion reaction with concomitant fluorescence "turned-on". With these new molecules in hand, we have developed a new approach for rapid, selective and fluorogenic labeling of endogenous protein in living cells. By using CA-II and eDHFR as model proteins, we demonstrated that subcellular localization of proteins can be precisely visualized by live-cell imaging and protein levels can be reliably quantified in multiple cell types using flow cytometry. Dynamic protein regulations such as hypoxia-induced CA-IX accumulation can also be detected. In addition, by two-photon excitation with an 800 nm laser, cell-selective labeling can also be achieved with spatially controlled irradiation. Our method circumvents the cytotoxicity of UV light and obviates the need for introducing external reporters with "click chemistries". We believe that this approach of fluorescence labeling of endogenous protein by bioorthogonal photo-irradiation opens up exciting opportunities for discoveries and mechanistic interrogation in chemical biology.
中文翻译:

用于活细胞中荧光蛋白标记的可见光和近红外光可激活重氮香豆素探针
活细胞中蛋白质的化学修饰可以让我们对支持动态细胞事件的分子相互作用进行有价值的了解。虽然基因工程方法通常是首选,但用化学方法在复杂的细胞内环境中选择性标记内源蛋白质是一个重大挑战。在这项研究中,我们报道了新型重氮香豆素化合物,可以通过可见光(430-490 nm)和近红外光(800 nm)照射光激活反应性卡宾中间体,随后可以与光发生插入反应伴随荧光“打开”。有了这些新分子,我们开发了一种对活细胞内源蛋白进行快速、选择性和荧光标记的新方法。通过使用 CA-II 和 eDHFR 作为模型蛋白,我们证明了可以通过活细胞成像精确地可视化蛋白质的亚细胞定位,并且可以使用流式细胞术可靠地量化多种细胞类型中的蛋白质水平。还可以检测动态蛋白质调节,例如缺氧诱导的 CA-IX 积累。此外,通过800 nm激光的双光子激发,还可以通过空间控制的照射来实现细胞选择性标记。我们的方法规避了紫外线的细胞毒性,并且无需引入具有“点击化学”的外部记者。我们相信,这种通过生物正交光照射荧光标记内源蛋白质的方法为化学生物学的发现和机制探究开辟了令人兴奋的机会。
更新日期:2020-09-01
中文翻译:

用于活细胞中荧光蛋白标记的可见光和近红外光可激活重氮香豆素探针
活细胞中蛋白质的化学修饰可以让我们对支持动态细胞事件的分子相互作用进行有价值的了解。虽然基因工程方法通常是首选,但用化学方法在复杂的细胞内环境中选择性标记内源蛋白质是一个重大挑战。在这项研究中,我们报道了新型重氮香豆素化合物,可以通过可见光(430-490 nm)和近红外光(800 nm)照射光激活反应性卡宾中间体,随后可以与光发生插入反应伴随荧光“打开”。有了这些新分子,我们开发了一种对活细胞内源蛋白进行快速、选择性和荧光标记的新方法。通过使用 CA-II 和 eDHFR 作为模型蛋白,我们证明了可以通过活细胞成像精确地可视化蛋白质的亚细胞定位,并且可以使用流式细胞术可靠地量化多种细胞类型中的蛋白质水平。还可以检测动态蛋白质调节,例如缺氧诱导的 CA-IX 积累。此外,通过800 nm激光的双光子激发,还可以通过空间控制的照射来实现细胞选择性标记。我们的方法规避了紫外线的细胞毒性,并且无需引入具有“点击化学”的外部记者。我们相信,这种通过生物正交光照射荧光标记内源蛋白质的方法为化学生物学的发现和机制探究开辟了令人兴奋的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号