当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Bioorthogonal Labeling Uncovers Endogenous Cotranslationally O-GlcNAc Modified Nascent Proteins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c04121 Yanping Zhu 1, 2 , Lianne I Willems 1 , Daniela Salas 1, 3 , Samy Cecioni 1 , Weifeng B Wu 2 , Leonard J Foster 3 , David J Vocadlo 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c04121 Yanping Zhu 1, 2 , Lianne I Willems 1 , Daniela Salas 1, 3 , Samy Cecioni 1 , Weifeng B Wu 2 , Leonard J Foster 3 , David J Vocadlo 1, 2
Affiliation
|
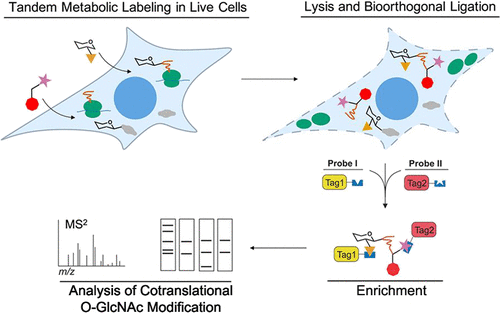
Hundreds of nuclear, cytoplasmic, and mitochondrial proteins within multicellular eukaryotes have hydroxyl groups of specific serine and threonine residues modified by the monosaccharide N-acetylglucosamine (GlcNAc). This modification, known as O-GlcNAc, has emerged as a central regulator of both cell physiology and human health. A key emerging function of O-GlcNAc appears to be to regulate cellular protein homeostasis. We previously showed, using overexpressed model proteins, that O-GlcNAc modification can occur cotranslationally and that this process prevents premature degradation of such nascent polypeptide chains. Here, we use tandem metabolic engineering strategies to label endogenously occurring nascent polypeptide chains within cells using O-propargyl-puromycin (OPP) and target the specific subset of nascent chains that are cotranslationally glycosylated with O-GlcNAc by metabolic saccharide engineering using tetra-O-acetyl-2-N-azidoacetyl-2-deoxy-d-galactopyranose (Ac4GalNAz). Using various combinations of sequential chemoselective ligation strategies, we go on to tag these analytes with a series of labels, allowing us to define conditions that enable their robust labeling. Two-step enrichment of these glycosylated nascent chains, combined with shotgun proteomics, allows us to identify a set of endogenous cotranslationally O-GlcNAc modified proteins. Using alternative targeted methods, we examine three of these identified proteins and further validate their cotranslational O-GlcNAcylation. These findings detail strategies to enable isolation and identification of extremely low abundance endogenous analytes present within complex protein mixtures. Moreover, this work opens the way to studies directed at understanding the roles of O-GlcNAc and other cotranslational protein modifications and should stimulate an improved understanding of the role of O-GlcNAc in cytoplasmic protein quality control and proteostasis.
中文翻译:

串联生物正交标记揭示内源性共翻译 O-GlcNAc 修饰的新生蛋白
多细胞真核生物中数百个核、细胞质和线粒体蛋白具有由单糖 N-乙酰氨基葡萄糖 (GlcNAc) 修饰的特定丝氨酸和苏氨酸残基的羟基。这种被称为 O-GlcNAc 的修饰已成为细胞生理学和人类健康的中心调节剂。O-GlcNAc 的一个关键新兴功能似乎是调节细胞蛋白质稳态。我们之前使用过表达的模型蛋白表明,O-GlcNAc 修饰可以协同翻译发生,并且该过程可防止这种新生多肽链过早降解。这里,我们使用串联代谢工程策略,使用 O-炔丙基嘌呤霉素 (OPP) 标记细胞内内源性发生的新生多肽链,并通过使用四-O-乙酰基的代谢糖工程来靶向与 O-GlcNAc 共翻译糖基化的新生链的特定子集-2-N-叠氮基乙酰基-2-脱氧-d-吡喃半乳糖 (Ac4GalNAz)。使用顺序化学选择性连接策略的各种组合,我们继续用一系列标签标记这些分析物,使我们能够定义能够对其进行可靠标记的条件。这些糖基化新生链的两步富集,结合鸟枪蛋白质组学,使我们能够鉴定一组内源性共翻译 O-GlcNAc 修饰的蛋白质。使用替代的靶向方法,我们检查了这些鉴定出的蛋白质中的三个,并进一步验证了它们的共翻译 O-GlcNAcylation。这些发现详述了能够分离和鉴定复杂蛋白质混合物中存在的极低丰度内源性分析物的策略。此外,这项工作为旨在了解 O-GlcNAc 和其他共翻译蛋白修饰作用的研究开辟了道路,并应促进对 O-GlcNAc 在细胞质蛋白质质量控制和蛋白质稳态中作用的更好理解。
更新日期:2020-09-01
中文翻译:

串联生物正交标记揭示内源性共翻译 O-GlcNAc 修饰的新生蛋白
多细胞真核生物中数百个核、细胞质和线粒体蛋白具有由单糖 N-乙酰氨基葡萄糖 (GlcNAc) 修饰的特定丝氨酸和苏氨酸残基的羟基。这种被称为 O-GlcNAc 的修饰已成为细胞生理学和人类健康的中心调节剂。O-GlcNAc 的一个关键新兴功能似乎是调节细胞蛋白质稳态。我们之前使用过表达的模型蛋白表明,O-GlcNAc 修饰可以协同翻译发生,并且该过程可防止这种新生多肽链过早降解。这里,我们使用串联代谢工程策略,使用 O-炔丙基嘌呤霉素 (OPP) 标记细胞内内源性发生的新生多肽链,并通过使用四-O-乙酰基的代谢糖工程来靶向与 O-GlcNAc 共翻译糖基化的新生链的特定子集-2-N-叠氮基乙酰基-2-脱氧-d-吡喃半乳糖 (Ac4GalNAz)。使用顺序化学选择性连接策略的各种组合,我们继续用一系列标签标记这些分析物,使我们能够定义能够对其进行可靠标记的条件。这些糖基化新生链的两步富集,结合鸟枪蛋白质组学,使我们能够鉴定一组内源性共翻译 O-GlcNAc 修饰的蛋白质。使用替代的靶向方法,我们检查了这些鉴定出的蛋白质中的三个,并进一步验证了它们的共翻译 O-GlcNAcylation。这些发现详述了能够分离和鉴定复杂蛋白质混合物中存在的极低丰度内源性分析物的策略。此外,这项工作为旨在了解 O-GlcNAc 和其他共翻译蛋白修饰作用的研究开辟了道路,并应促进对 O-GlcNAc 在细胞质蛋白质质量控制和蛋白质稳态中作用的更好理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号