当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent‐free synthesis of propargylamines via A3 coupling reaction and organic pollutant degradation in aqueous condition using Cu/C catalyst
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-09-01 , DOI: 10.1002/aoc.5986 Pramod V. Rathod 1 , John Marc C. Puguan 1 , Hern Kim 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-09-01 , DOI: 10.1002/aoc.5986 Pramod V. Rathod 1 , John Marc C. Puguan 1 , Hern Kim 1
Affiliation
|
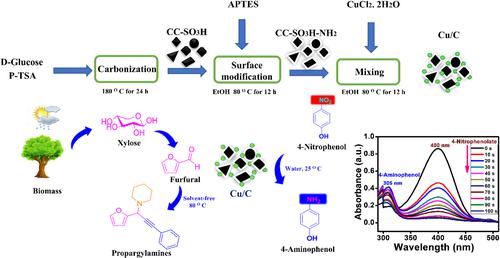
The present report focuses on the efficient and operationally simple synthesis of biomass‐derived carbon as support to immobilize copper particles as a catalyst for the one‐pot synthesis of propargylamines from furfural via the A3 coupling reaction. This new catalyst showed remarkable catalytic performance leading to a 97% yield within 5 h at 80 °C using 5 mg (0.0022 mmol Cu) of Cu/C catalyst under solvent‐free condition. Moreover, nitro‐substituted compounds such as 4‐nitrophenol (4‐NP) are highly toxic and not easily degradable. Hence, a quick and effective method is required to neutralize these toxic compounds. The synthesized active support Cu/C catalyst having various electron‐donating groups containing small amounts of Cu plays an essential role in the catalytic reduction of 4‐NP (0.1 g). Using only 3 mg (0.0013 mmol Cu) of Cu/C catalyst and NaBH4 (10 mmol), a 99% yield (100% selectivity) in the aqueous condition at 25 °C was achieved. The catalytic reduction follows the pseudo‐first‐order kinetics with reaction rate constant of 0.028 s−1. Moreover, results demonstrate that the Cu/C catalyst has superior catalytic activity due to the presence of electron‐donating molecules such as O, S, and N atoms, which enable synergistic effect in enhancing the overall catalytic performance. Notably, the recoverability and recyclability of the synthesized catalyst were evaluated for up to four cycles, which confirmed its stability in these cycles.
中文翻译:

使用Cu / C催化剂通过A3偶联反应无溶剂合成炔丙胺并在水性条件下降解有机污染物
本报告着重于高效且操作简单的合成生物质衍生碳作为载体,以固定铜颗粒作为催化剂,通过A 3从糠醛一锅合成炔丙基胺。偶联反应。这种新型催化剂表现出了出色的催化性能,在无溶剂条件下使用5 mg(0.0022 mmol Cu)的Cu / C催化剂在80°C下5h内收率达97%。此外,硝基取代的化合物(例如4-硝基苯酚(4-NP))剧毒且不易降解。因此,需要一种快速有效的方法来中和这些有毒化合物。合成的具有各种电子给体基团且含有少量Cu的活性载体Cu / C催化剂在4-NP(0.1 g)的催化还原中起着至关重要的作用。仅使用3 mg(0.0013 mmol Cu)的Cu / C催化剂和NaBH 4(10mmol),在25℃的水性条件下获得99%的收率(100%的选择性)。催化还原遵循拟一级动力学,反应速率常数为0.028 s -1。此外,结果表明,由于存在电子给体分子(例如O,S和N原子),Cu / C催化剂具有优异的催化活性,从而在增强整体催化性能方面具有协同作用。值得注意的是,对合成催化剂的可回收性和可循环性进行了多达四个循环的评价,这证实了其在这些循环中的稳定性。
更新日期:2020-11-06
中文翻译:

使用Cu / C催化剂通过A3偶联反应无溶剂合成炔丙胺并在水性条件下降解有机污染物
本报告着重于高效且操作简单的合成生物质衍生碳作为载体,以固定铜颗粒作为催化剂,通过A 3从糠醛一锅合成炔丙基胺。偶联反应。这种新型催化剂表现出了出色的催化性能,在无溶剂条件下使用5 mg(0.0022 mmol Cu)的Cu / C催化剂在80°C下5h内收率达97%。此外,硝基取代的化合物(例如4-硝基苯酚(4-NP))剧毒且不易降解。因此,需要一种快速有效的方法来中和这些有毒化合物。合成的具有各种电子给体基团且含有少量Cu的活性载体Cu / C催化剂在4-NP(0.1 g)的催化还原中起着至关重要的作用。仅使用3 mg(0.0013 mmol Cu)的Cu / C催化剂和NaBH 4(10mmol),在25℃的水性条件下获得99%的收率(100%的选择性)。催化还原遵循拟一级动力学,反应速率常数为0.028 s -1。此外,结果表明,由于存在电子给体分子(例如O,S和N原子),Cu / C催化剂具有优异的催化活性,从而在增强整体催化性能方面具有协同作用。值得注意的是,对合成催化剂的可回收性和可循环性进行了多达四个循环的评价,这证实了其在这些循环中的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号