当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lysozyme crystals dyed with bromophenol blue: where has the dye gone?
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-09-02 , DOI: 10.1107/s2059798320008803 Marina Plaza-Garrido 1 , M Carmen Salinas-Garcia 1 , Daniel Alba-Elena 1 , Jose C Martínez 2 , Ana Camara-Artigas 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-09-02 , DOI: 10.1107/s2059798320008803 Marina Plaza-Garrido 1 , M Carmen Salinas-Garcia 1 , Daniel Alba-Elena 1 , Jose C Martínez 2 , Ana Camara-Artigas 1
Affiliation
|
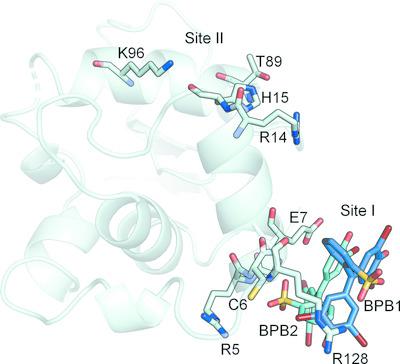
Protein crystals can easily be coloured by adding dyes to their mother liquor, but most structures of these protein–dye complexes remain unsolved. Here, structures of lysozyme in complex with bromophenol blue obtained by soaking orthorhombic and tetragonal crystals in a saturated solution of the dye at different pH values from 5.0 to 7.5 are reported. Two different binding sites can be found in the lysozyme–bromophenol blue crystals: binding site I is located near the amino‐ and carboxyl‐termini, while binding site II is located adjacent to helices α1 (residues 4–15) and α3 (residues 88–100). In the orthorhombic crystals soaked at pH 7.0, binding of the dye takes place in both sites without significant changes in the unit cell. However, soaking tetragonal crystals with bromophenol blue results in two different complexes. Crystals soaked at pH 5.5 (HEWL‐T1) show a single dye molecule bound to site II, and the crystals belong to space group P43212 without significant changes in the unit cell (a = b = 78.50, c = 37.34 Å). On the other hand, crystals soaked at pH 6.5 in the presence of imidazole (HEWL‐T2) show up to eight molecules of the dye bound to site II, and display changes in space group (P212121) and unit cell (a = 38.00, b = 76.65, c = 84.86 Å). In all of the structures, the dye molecules are placed at the surface of the protein near to positively charged residues accessible through the main solvent channels of the crystal. Differences in the arrangement of the dye molecules at the surface of the protein suggest that the binding is not specific and is mainly driven by electrostatic interactions.
中文翻译:

用溴酚蓝染色的溶菌酶晶体:染料去了哪里?
通过在母液中添加染料可以轻松地对蛋白质晶体进行着色,但是这些蛋白质-染料复合物的大多数结构仍未溶解。在此,报道了通过将正交晶体和四方晶体在5.0至7.5的不同pH值下浸入染料的饱和溶液中而获得的与溴酚蓝配合的溶菌酶的结构。在溶菌酶-溴酚蓝晶体中可以找到两个不同的结合位点:结合位点I位于氨基和羧基末端附近,而结合位点II位于螺旋α1(残基4-15)和α3(残基88)附近。 –100)。在pH 7.0下浸泡的正交晶体中,染料的结合发生在两个位点,而晶胞没有明显变化。但是,用溴酚蓝浸泡四方晶体会产生两种不同的络合物。晶体在pH 5下浸泡。P 4 3 2 1 2在晶胞中无明显变化(a = b = 78.50,c = 37.34Å)。另一方面,在咪唑(HEWL-T2)存在下在pH 6.5下浸泡的晶体最多显示八个与II位结合的染料分子,并显示出空间群(P 2 1 2 1 2 1)和单位的变化。单元格(a = 38.00,b = 76.65,c = 84.86Å)。在所有结构中,染料分子都位于蛋白质表面,靠近可通过晶体主要溶剂通道进入的带正电荷的残基。蛋白质表面染料分子排列的差异表明结合不是特异性的,主要是由静电相互作用驱动的。
更新日期:2020-09-02
中文翻译:

用溴酚蓝染色的溶菌酶晶体:染料去了哪里?
通过在母液中添加染料可以轻松地对蛋白质晶体进行着色,但是这些蛋白质-染料复合物的大多数结构仍未溶解。在此,报道了通过将正交晶体和四方晶体在5.0至7.5的不同pH值下浸入染料的饱和溶液中而获得的与溴酚蓝配合的溶菌酶的结构。在溶菌酶-溴酚蓝晶体中可以找到两个不同的结合位点:结合位点I位于氨基和羧基末端附近,而结合位点II位于螺旋α1(残基4-15)和α3(残基88)附近。 –100)。在pH 7.0下浸泡的正交晶体中,染料的结合发生在两个位点,而晶胞没有明显变化。但是,用溴酚蓝浸泡四方晶体会产生两种不同的络合物。晶体在pH 5下浸泡。P 4 3 2 1 2在晶胞中无明显变化(a = b = 78.50,c = 37.34Å)。另一方面,在咪唑(HEWL-T2)存在下在pH 6.5下浸泡的晶体最多显示八个与II位结合的染料分子,并显示出空间群(P 2 1 2 1 2 1)和单位的变化。单元格(a = 38.00,b = 76.65,c = 84.86Å)。在所有结构中,染料分子都位于蛋白质表面,靠近可通过晶体主要溶剂通道进入的带正电荷的残基。蛋白质表面染料分子排列的差异表明结合不是特异性的,主要是由静电相互作用驱动的。










































 京公网安备 11010802027423号
京公网安备 11010802027423号