当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recognition of an α-helical hairpin in P22 large terminase by a synthetic antibody fragment.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-09-02 , DOI: 10.1107/s2059798320009912 Ravi K Lokareddy 1 , Ying Hui Ko 1 , Nathaniel Hong 1 , Steven G Doll 1 , Marcin Paduch 2 , Michael Niederweis 3 , Anthony A Kossiakoff 2 , Gino Cingolani 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-09-02 , DOI: 10.1107/s2059798320009912 Ravi K Lokareddy 1 , Ying Hui Ko 1 , Nathaniel Hong 1 , Steven G Doll 1 , Marcin Paduch 2 , Michael Niederweis 3 , Anthony A Kossiakoff 2 , Gino Cingolani 1
Affiliation
|
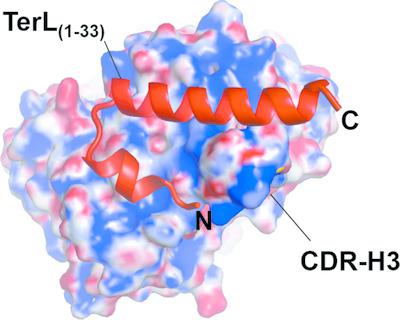
The genome‐packaging motor of tailed bacteriophages and herpesviruses is a multisubunit protein complex formed by several copies of a large (TerL) and a small (TerS) terminase subunit. The motor assembles transiently at the portal protein vertex of an empty precursor capsid to power the energy‐dependent packaging of viral DNA. Both the ATPase and nuclease activities associated with genome packaging reside in TerL. Structural studies of TerL from bacteriophage P22 have been hindered by the conformational flexibility of this enzyme and its susceptibility to proteolysis. Here, an unbiased, synthetic phage‐display Fab library was screened and a panel of high‐affinity Fabs against P22 TerL were identified. This led to the discovery of a recombinant antibody fragment, Fab4, that binds a 33‐amino‐acid α‐helical hairpin at the N‐terminus of TerL with an equilibrium dissociation constant Kd of 71.5 nM. A 1.51 Å resolution crystal structure of Fab4 bound to the TerL epitope (TLE) together with a 1.15 Å resolution crystal structure of the unliganded Fab4, which is the highest resolution ever achieved for a Fab, elucidate the principles governing the recognition of this novel helical epitope. TLE adopts two different conformations in the asymmetric unit and buries as much as 1250 Å2 of solvent‐accessible surface in Fab4. TLE recognition is primarily mediated by conformational changes in the third complementarity‐determining region of the Fab4 heavy chain (CDR H3) that take place upon epitope binding. It is demonstrated that TLE can be introduced genetically at the N‐terminus of a target protein, where it retains high‐affinity binding to Fab4.
中文翻译:

通过合成抗体片段识别 P22 大终止酶中的 α-螺旋发夹。
有尾噬菌体和疱疹病毒的基因组包装马达是一个多亚基蛋白复合物,由多个大(TerL)和一个小(TerS)终止酶亚基的拷贝形成。马达在空前体衣壳的门蛋白顶点瞬时组装,为病毒 DNA 的能量依赖性包装提供动力。与基因组包装相关的 ATP 酶和核酸酶活性都位于 TerL 中。来自噬菌体 P22 的 TerL 的结构研究因该酶的构象灵活性及其对蛋白水解的敏感性而受到阻碍。在这里,筛选了一个公正的合成噬菌体展示 Fab 文库,并鉴定了一组针对 P22 TerL 的高亲和力 Fab。这导致了重组抗体片段 Fab4 的发现,它在 TerL N 末端结合 33 个氨基酸的 α 螺旋发夹,平衡解离常数K d为 71.5 n M。与 TerL 表位 (TLE) 结合的 Fab4 的 1.51 Å 分辨率晶体结构以及未配体 Fab4 的 1.15 Å 分辨率晶体结构(这是 Fab 迄今为止实现的最高分辨率),阐明了识别这种新型螺旋的原理表位。TLE 在不对称单元中采用两种不同的构象,并在 Fab4 中埋藏了多达 1250 Å 2的溶剂可及表面。TLE 识别主要由表位结合时发生的 Fab4 重链第三个互补决定区 (CDR H3) 的构象变化介导。事实证明,TLE 可以通过基因方式引入目标蛋白的 N 末端,并保留与 Fab4 的高亲和力结合。
更新日期:2020-09-02
中文翻译:

通过合成抗体片段识别 P22 大终止酶中的 α-螺旋发夹。
有尾噬菌体和疱疹病毒的基因组包装马达是一个多亚基蛋白复合物,由多个大(TerL)和一个小(TerS)终止酶亚基的拷贝形成。马达在空前体衣壳的门蛋白顶点瞬时组装,为病毒 DNA 的能量依赖性包装提供动力。与基因组包装相关的 ATP 酶和核酸酶活性都位于 TerL 中。来自噬菌体 P22 的 TerL 的结构研究因该酶的构象灵活性及其对蛋白水解的敏感性而受到阻碍。在这里,筛选了一个公正的合成噬菌体展示 Fab 文库,并鉴定了一组针对 P22 TerL 的高亲和力 Fab。这导致了重组抗体片段 Fab4 的发现,它在 TerL N 末端结合 33 个氨基酸的 α 螺旋发夹,平衡解离常数K d为 71.5 n M。与 TerL 表位 (TLE) 结合的 Fab4 的 1.51 Å 分辨率晶体结构以及未配体 Fab4 的 1.15 Å 分辨率晶体结构(这是 Fab 迄今为止实现的最高分辨率),阐明了识别这种新型螺旋的原理表位。TLE 在不对称单元中采用两种不同的构象,并在 Fab4 中埋藏了多达 1250 Å 2的溶剂可及表面。TLE 识别主要由表位结合时发生的 Fab4 重链第三个互补决定区 (CDR H3) 的构象变化介导。事实证明,TLE 可以通过基因方式引入目标蛋白的 N 末端,并保留与 Fab4 的高亲和力结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号