当前位置:
X-MOL 学术
›
NMR Biomed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hemorrhage promotes chronic adverse remodeling in acute myocardial infarction: a T1 , T2 and BOLD study.
NMR in Biomedicine ( IF 2.9 ) Pub Date : 2020-09-01 , DOI: 10.1002/nbm.4404 Stephania Assimopoulos 1 , Nancy Shie 2 , Venkat Ramanan 2 , Xiuling Qi 2 , Jennifer Barry 2 , Bradley H Strauss 3 , Graham A Wright 1, 2, 3 , Nilesh R Ghugre 1, 2, 3
NMR in Biomedicine ( IF 2.9 ) Pub Date : 2020-09-01 , DOI: 10.1002/nbm.4404 Stephania Assimopoulos 1 , Nancy Shie 2 , Venkat Ramanan 2 , Xiuling Qi 2 , Jennifer Barry 2 , Bradley H Strauss 3 , Graham A Wright 1, 2, 3 , Nilesh R Ghugre 1, 2, 3
Affiliation
|
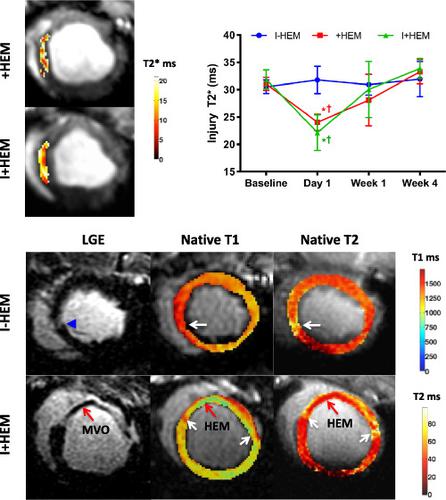
Hemorrhage is recognized as a new independent predictor of adverse outcomes following acute myocardial infarction. However, the mechanisms of its effects are less understood. The aim of our study was to probe the downstream impact of hemorrhage towards chronic remodeling, including inflammation, vasodilator function and matrix alterations in an experimental model of hemorrhage. Myocardial hemorrhage was induced in the porcine heart by intracoronary injection of collagenase. Animals (N = 18) were subjected to coronary occlusion followed by reperfusion in three groups (six/group): 8 min ischemia with hemorrhage (+HEM), 45 min infarction with no hemorrhage (I − HEM) and 45 min infarction with hemorrhage (I + HEM). MRI was performed up to 4 weeks after intervention. Cardiac function, edema (T2, T1), hemorrhage (T2*), vasodilator function (T2 BOLD), infarction and microvascular obstruction (MVO) and partition coefficient (pre‐ and post‐contrast T1) were computed. Hemorrhage was induced only in the +HEM and I + HEM groups on Day 1 (low T2* values). Infarct size was the greatest in the I + HEM group, while the +HEM group showed no observable infarct. MVO was seen only in the I + HEM group, with a 40% occurrence rate. Function was compromised and ventricular volume was enlarged only in the hemorrhage groups and not in the ischemia‐alone group. In the infarct zone, edema and matrix expansion were the greatest in the I + HEM group. In the remote myocardium, T2 elevation and matrix expansion associated with a transient vasodilator dysfunction were observed in the hemorrhage groups but not in the ischemia‐alone group. Our study demonstrates that the introduction of myocardial hemorrhage at reperfusion results in greater myocardial damage, upregulated inflammation, chronic adverse remodeling and remote myocardial alterations beyond the effects of the initial ischemic insult. A systematic understanding of the consequences of hemorrhage will potentially aid in the identification of novel therapeutics for high‐risk patients progressing towards heart failure.
中文翻译:

出血促进急性心肌梗塞中的慢性不良重塑:T1、T2 和 BOLD 研究。
出血被认为是急性心肌梗死后不良结局的一个新的独立预测因子。然而,对其影响的机制知之甚少。我们研究的目的是探讨出血对慢性重塑的下游影响,包括出血实验模型中的炎症、血管扩张功能和基质改变。通过冠状动脉内注射胶原酶在猪心脏中诱导心肌出血。动物(N = 18)接受冠状动脉闭塞,然后分三组(每组 6 只)进行再灌注:8 分钟缺血出血 (+HEM)、45 分钟无出血梗塞 (I - HEM) 和 45 分钟梗塞出血(我+下摆)。MRI 在干预后 4 周内进行。心功能、水肿(T2 , T 1 )、出血 ( T 2 *)、血管舒张功能 ( T 2 BOLD)、梗死和微血管阻塞 (MVO) 和分配系数(对比前和对比后T 1)被计算。在第 1 天(低T 2* 值)。I + HEM 组的梗塞面积最大,而 +HEM 组未显示可观察到的梗塞。MVO仅见于I+HEM组,发生率为40%。仅在出血组而非单独缺血组中,功能受损且心室容积增大。在梗死区,I + HEM 组的水肿和基质膨胀最大。在远端心肌中,T 2在出血组中观察到与短暂血管扩张功能障碍相关的升高和基质扩张,但在单独缺血组中未观察到。我们的研究表明,在再灌注时引入心肌出血会导致更大的心肌损伤、炎症上调、慢性不良重构和超出初始缺血性损伤影响的远程心肌改变。对出血后果的系统理解可能有助于为进展为心力衰竭的高危患者确定新的治疗方法。
更新日期:2020-09-01
中文翻译:

出血促进急性心肌梗塞中的慢性不良重塑:T1、T2 和 BOLD 研究。
出血被认为是急性心肌梗死后不良结局的一个新的独立预测因子。然而,对其影响的机制知之甚少。我们研究的目的是探讨出血对慢性重塑的下游影响,包括出血实验模型中的炎症、血管扩张功能和基质改变。通过冠状动脉内注射胶原酶在猪心脏中诱导心肌出血。动物(N = 18)接受冠状动脉闭塞,然后分三组(每组 6 只)进行再灌注:8 分钟缺血出血 (+HEM)、45 分钟无出血梗塞 (I - HEM) 和 45 分钟梗塞出血(我+下摆)。MRI 在干预后 4 周内进行。心功能、水肿(T2 , T 1 )、出血 ( T 2 *)、血管舒张功能 ( T 2 BOLD)、梗死和微血管阻塞 (MVO) 和分配系数(对比前和对比后T 1)被计算。在第 1 天(低T 2* 值)。I + HEM 组的梗塞面积最大,而 +HEM 组未显示可观察到的梗塞。MVO仅见于I+HEM组,发生率为40%。仅在出血组而非单独缺血组中,功能受损且心室容积增大。在梗死区,I + HEM 组的水肿和基质膨胀最大。在远端心肌中,T 2在出血组中观察到与短暂血管扩张功能障碍相关的升高和基质扩张,但在单独缺血组中未观察到。我们的研究表明,在再灌注时引入心肌出血会导致更大的心肌损伤、炎症上调、慢性不良重构和超出初始缺血性损伤影响的远程心肌改变。对出血后果的系统理解可能有助于为进展为心力衰竭的高危患者确定新的治疗方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号