当前位置:
X-MOL 学术
›
J. Inherit. Metab. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional evaluation of 16 SCHAD missense variants: Only amino acid substitutions causing congenital hyperinsulinism of infancy lead to loss‐of‐function phenotypes in vitro
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-09-02 , DOI: 10.1002/jimd.12309 Kelly Velasco 1 , Johanna L St-Louis 1 , Henrikke N Hovland 1 , Nels Thompson 1 , Åsta Ottesen 1 , Man Hung Choi 1, 2 , Line Pedersen 1 , Pål R Njølstad 3, 4 , Thomas Arnesen 5, 6, 7 , Karianne Fjeld 1, 8 , Ingvild Aukrust 3, 8 , Line M Myklebust 5, 6 , Anders Molven 1, 2, 3
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-09-02 , DOI: 10.1002/jimd.12309 Kelly Velasco 1 , Johanna L St-Louis 1 , Henrikke N Hovland 1 , Nels Thompson 1 , Åsta Ottesen 1 , Man Hung Choi 1, 2 , Line Pedersen 1 , Pål R Njølstad 3, 4 , Thomas Arnesen 5, 6, 7 , Karianne Fjeld 1, 8 , Ingvild Aukrust 3, 8 , Line M Myklebust 5, 6 , Anders Molven 1, 2, 3
Affiliation
|
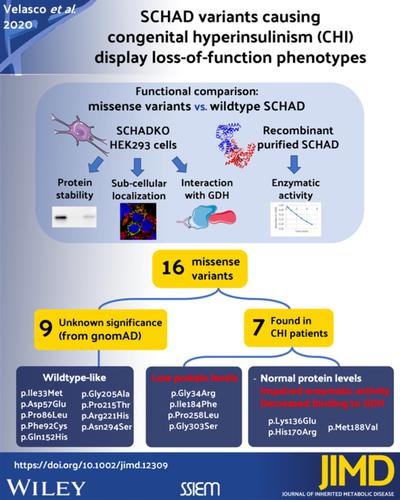
Short‐chain 3‐hydroxyacyl‐CoA dehydrogenase (SCHAD), encoded by the HADH gene, is a ubiquitously expressed mitochondrial enzyme involved in fatty acid oxidation. This protein also plays a role in insulin secretion as recessive HADH mutations cause congenital hyperinsulinism of infancy (CHI) via loss of an inhibitory interaction with glutamate dehydrogenase (GDH). Here, we present a functional evaluation of 16 SCHAD missense variants identified either in CHI patients or by high‐throughput sequencing projects in various populations. To avoid interactions with endogenously produced SCHAD protein, we assessed protein stability, subcellular localization, and GDH interaction in a SCHAD knockout HEK293 cell line constructed by CRISPR‐Cas9 methodology. We also established methods for efficient SCHAD expression and purification in E. coli, and tested enzymatic activity of the variants. Our analyses showed that rare variants of unknown significance identified in populations generally had similar properties as normal SCHAD. However, the CHI‐associated variants p.Gly34Arg, p.Ile184Phe, p.Pro258Leu, and p.Gly303Ser were unstable with low protein levels detectable when expressed in HEK293 cells. Moreover, CHI variants p.Lys136Glu, p.His170Arg, and p.Met188Val presented normal protein levels but displayed clearly impaired enzymatic activity in vitro, and their interaction with GDH appeared reduced. Our results suggest that pathogenic missense variants of SCHAD either make the protein target of a post‐translational quality control system or can impair the function of SCHAD without influencing its steady‐state protein level. We did not find any evidence that rare SCHAD missense variants observed only in the general population and not in CHI patients are functionally affected.
中文翻译:

16 种 SCHAD 错义变体的功能评估:仅导致婴儿先天性高胰岛素血症的氨基酸替代导致体外功能丧失表型
由HADH基因编码的短链 3-羟酰基辅酶 A 脱氢酶 (SCHAD)是一种普遍表达的参与脂肪酸氧化的线粒体酶。这种蛋白质也作为隐性HADH在胰岛素分泌中起作用突变通过失去与谷氨酸脱氢酶 (GDH) 的抑制性相互作用而导致婴儿先天性高胰岛素血症 (CHI)。在这里,我们对在 CHI 患者中或通过高通量测序项目在不同人群中发现的 16 种 SCHAD 错义变异进行了功能评估。为了避免与内源性产生的 SCHAD 蛋白相互作用,我们评估了通过 CRISPR-Cas9 方法构建的 SCHAD 敲除 HEK293 细胞系中的蛋白质稳定性、亚细胞定位和 GDH 相互作用。我们还建立了在大肠杆菌中高效表达和纯化 SCHAD 的方法,并测试了变体的酶活性。我们的分析表明,在人群中发现的意义不明的罕见变异通常与正常 SCHAD 具有相似的特性。然而,CHI 相关变体 p.Gly34Arg、p.Ile184Phe、p.Pro258Leu 和 p.Gly303Ser 不稳定,在 HEK293 细胞中表达时可检测到低蛋白水平。此外,CHI 变体 p.Lys136Glu、p.His170Arg 和 p.Met188Val 呈现正常的蛋白质水平,但在体外显示出明显受损的酶活性,并且它们与 GDH 的相互作用似乎降低。我们的结果表明,SCHAD 的致病性错义变异要么成为翻译后质量控制系统的蛋白质靶标,要么在不影响其稳态蛋白质水平的情况下损害 SCHAD 的功能。
更新日期:2020-09-02
中文翻译:

16 种 SCHAD 错义变体的功能评估:仅导致婴儿先天性高胰岛素血症的氨基酸替代导致体外功能丧失表型
由HADH基因编码的短链 3-羟酰基辅酶 A 脱氢酶 (SCHAD)是一种普遍表达的参与脂肪酸氧化的线粒体酶。这种蛋白质也作为隐性HADH在胰岛素分泌中起作用突变通过失去与谷氨酸脱氢酶 (GDH) 的抑制性相互作用而导致婴儿先天性高胰岛素血症 (CHI)。在这里,我们对在 CHI 患者中或通过高通量测序项目在不同人群中发现的 16 种 SCHAD 错义变异进行了功能评估。为了避免与内源性产生的 SCHAD 蛋白相互作用,我们评估了通过 CRISPR-Cas9 方法构建的 SCHAD 敲除 HEK293 细胞系中的蛋白质稳定性、亚细胞定位和 GDH 相互作用。我们还建立了在大肠杆菌中高效表达和纯化 SCHAD 的方法,并测试了变体的酶活性。我们的分析表明,在人群中发现的意义不明的罕见变异通常与正常 SCHAD 具有相似的特性。然而,CHI 相关变体 p.Gly34Arg、p.Ile184Phe、p.Pro258Leu 和 p.Gly303Ser 不稳定,在 HEK293 细胞中表达时可检测到低蛋白水平。此外,CHI 变体 p.Lys136Glu、p.His170Arg 和 p.Met188Val 呈现正常的蛋白质水平,但在体外显示出明显受损的酶活性,并且它们与 GDH 的相互作用似乎降低。我们的结果表明,SCHAD 的致病性错义变异要么成为翻译后质量控制系统的蛋白质靶标,要么在不影响其稳态蛋白质水平的情况下损害 SCHAD 的功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号