当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low Temperature Hydrothermal Method for Synthesis of Crystalline Fe2O3 and their Oxygen Evolution Performance
Electroanalysis ( IF 2.7 ) Pub Date : 2020-09-01 , DOI: 10.1002/elan.202060146 Supriya Rana 1, 2 , Krishna K. Yadav 1 , Kritika Sood 1 , Ankush 1 , S. K. Mehta 2 , Menaka Jha 1, 3
Electroanalysis ( IF 2.7 ) Pub Date : 2020-09-01 , DOI: 10.1002/elan.202060146 Supriya Rana 1, 2 , Krishna K. Yadav 1 , Kritika Sood 1 , Ankush 1 , S. K. Mehta 2 , Menaka Jha 1, 3
Affiliation
|
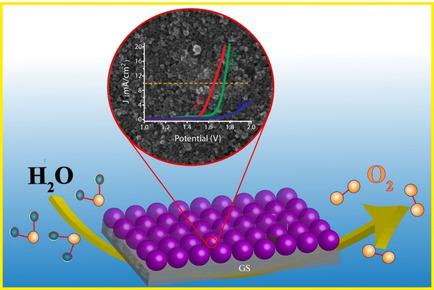
Hydrothermal method for the synthesis of hematite (α‐Fe2O3) has been done at 120 °C. Morphological study reveals the formation of spherical nanoparticles of average size ∼10 nm. The prepared electrode (Fe2O3 dropcosted over graphite sheet) used as a working electrode for the oxygen evolution reaction in 1 M KOH. The linear sweep voltammetry confirms that the as‐prepared electrode requires 340 mV potential to reach a current density of 1 mA/cm2 and 510 mV potential for 10 mA/cm2. Further, the kinetics of electrocatalyst has been investigated using Tafel slope, mass activity and Turn over frequency.
中文翻译:

低温水热法合成Fe2O3晶体及其析氧性能
为赤铁矿(α-Fe的合成水热法2 ö 3)已在120℃下被完成。形态学研究表明形成了平均粒径约为10 nm的球形纳米颗粒。所制备的电极(在石墨片上的Fe 2 O 3成本下降)用作在1 M KOH中进行氧释放反应的工作电极。线性扫描伏安法证实,所准备的电极需要340 mV的电势才能达到1 mA / cm 2的电流密度,而对于10 mA / cm 2的电势需要510 mV的电势。此外,已经使用Tafel斜率,质量活性和翻转频率研究了电催化剂的动力学。
更新日期:2020-09-01
中文翻译:

低温水热法合成Fe2O3晶体及其析氧性能
为赤铁矿(α-Fe的合成水热法2 ö 3)已在120℃下被完成。形态学研究表明形成了平均粒径约为10 nm的球形纳米颗粒。所制备的电极(在石墨片上的Fe 2 O 3成本下降)用作在1 M KOH中进行氧释放反应的工作电极。线性扫描伏安法证实,所准备的电极需要340 mV的电势才能达到1 mA / cm 2的电流密度,而对于10 mA / cm 2的电势需要510 mV的电势。此外,已经使用Tafel斜率,质量活性和翻转频率研究了电催化剂的动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号