当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Conformational States of Neurotensin Receptor 1 Variants by NMR Site-Directed Methyl Labeling.
ChemBioChem ( IF 3.2 ) Pub Date : 2020-09-02 , DOI: 10.1002/cbic.202000541 Inguna Goba 1 , David Goricanec 2 , Dominik Schum 2 , Matthias Hillenbrand 3 , Andreas Plückthun 3 , Franz Hagn 1, 2
ChemBioChem ( IF 3.2 ) Pub Date : 2020-09-02 , DOI: 10.1002/cbic.202000541 Inguna Goba 1 , David Goricanec 2 , Dominik Schum 2 , Matthias Hillenbrand 3 , Andreas Plückthun 3 , Franz Hagn 1, 2
Affiliation
|
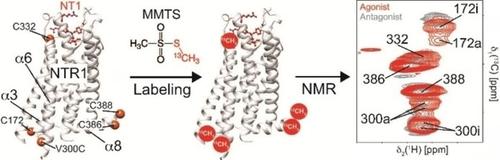
Active or inactive? We used NMR to probe the structural states of stabilized neurotensin receptor (NTR1) variants in complex with different ligands by chemical modification of surface‐exposed cysteine residues. This protocol was further used to design NTR1 variants with wild‐type‐like conformational switching properties that can be produced in bacterial hosts.

中文翻译:

通过 NMR 定点甲基标记探测神经降压素受体 1 变体的构象状态。
主动还是不主动?我们使用 NMR 通过化学修饰表面暴露的半胱氨酸残基来探测与不同配体复合的稳定神经降压素受体 (NTR1) 变体的结构状态。该协议进一步用于设计具有野生型样构象转换特性的 NTR1 变体,这些变体可以在细菌宿主中产生。

更新日期:2020-09-02

中文翻译:

通过 NMR 定点甲基标记探测神经降压素受体 1 变体的构象状态。
主动还是不主动?我们使用 NMR 通过化学修饰表面暴露的半胱氨酸残基来探测与不同配体复合的稳定神经降压素受体 (NTR1) 变体的结构状态。该协议进一步用于设计具有野生型样构象转换特性的 NTR1 变体,这些变体可以在细菌宿主中产生。




























 京公网安备 11010802027423号
京公网安备 11010802027423号